What Is The Electron Configuration For Helium
listenit
Mar 22, 2025 · 6 min read
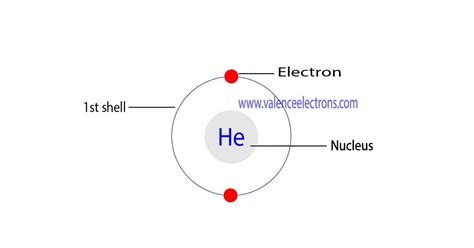
Table of Contents
What is the Electron Configuration for Helium? A Deep Dive into the Noble Gas
Helium, the second element on the periodic table, is a fascinating subject for understanding fundamental concepts in chemistry and physics. Its simplicity, being the second lightest element, belies its importance in various scientific fields and its unique properties. A crucial aspect of understanding helium's behavior lies in its electron configuration, a concept we'll explore in detail in this article.
Understanding Electron Configuration
Before delving into the specifics of helium's electron configuration, let's establish a foundational understanding of what electron configuration represents. In essence, an electron configuration describes the arrangement of electrons within the electron shells and subshells of an atom. This arrangement dictates an atom's chemical properties, its reactivity, and its behavior in various environments. It's governed by several principles:
The Aufbau Principle
The Aufbau principle, which translates to "building-up" principle, dictates that electrons fill the lowest energy levels first. Think of it like filling a container from the bottom up; you wouldn't start filling the top until the bottom is full. Electrons occupy orbitals with the lowest possible energy levels before moving to higher energy levels.
Hund's Rule
Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This is because electrons, possessing a negative charge, repel each other. They prefer to be alone in an orbital if possible, minimizing electron-electron repulsion.
The Pauli Exclusion Principle
The Pauli Exclusion Principle postulates that no two electrons in an atom can have the same set of four quantum numbers. This principle is crucial because it limits the number of electrons that can occupy a given orbital. Each orbital can hold a maximum of two electrons, but they must have opposite spins (represented as +1/2 and -1/2).
Helium's Electron Configuration: 1s²
Now, let's apply these principles to helium. Helium has an atomic number of 2, meaning it possesses two protons and, in a neutral atom, two electrons. Its electron configuration is concisely written as 1s². Let's break this down:
-
1: This refers to the principal quantum number (n), representing the energy level or shell. 1 indicates the first energy level, which is closest to the nucleus.
-
s: This represents the azimuthal quantum number (l), specifying the subshell. The 's' subshell is a spherical orbital, which can hold a maximum of two electrons.
-
²: This superscript indicates the number of electrons occupying the 1s subshell. In helium's case, both electrons fill the 1s orbital, one with spin +1/2 and the other with spin -1/2, completely filling the first energy level.
Why Helium's Configuration is Significant
Helium's simple electron configuration is the key to understanding its unique properties:
Noble Gas Configuration: Inertness and Stability
Helium's fully filled 1s orbital results in a remarkably stable electron configuration, characteristic of noble gases. This complete outermost electron shell makes helium extremely unreactive, chemically inert. It rarely forms chemical bonds with other elements, unlike many other elements that readily react to achieve a stable electron configuration. This inertness is what makes helium so useful in various applications, such as in balloons and protective atmospheres.
Low Boiling Point and Density: Quantum Effects
The weak interatomic forces in helium, a direct consequence of its filled electron shell, lead to an exceptionally low boiling point and density. It remains a gas even at extremely low temperatures, a property exploited in cryogenics. Quantum effects significantly influence helium's behavior at low temperatures, highlighting the importance of understanding its electronic structure.
Unique Spectroscopic Properties
The energy difference between helium's ground state (1s²) and its excited states is significant, resulting in unique spectral lines when helium atoms transition between these energy levels. These characteristic spectral lines are used in various analytical techniques, including spectroscopy, for identifying the presence of helium in different environments.
Helium's Applications: A Consequence of its Electron Configuration
The unique properties stemming from helium's electron configuration have led to its widespread use in a multitude of applications:
Cryogenics
Helium's exceptionally low boiling point makes it an essential coolant in cryogenic applications, such as superconducting magnets used in MRI machines and scientific research involving extremely low temperatures.
Welding and Leak Detection
Helium's inert nature is utilized in welding processes to create a protective atmosphere around the weld, preventing oxidation and other undesirable reactions. Its ability to diffuse readily through small leaks makes it ideal for detecting leaks in various systems.
Balloons and Airships
Helium's low density and inertness make it an excellent lifting gas for balloons and airships, offering a safer alternative to the highly flammable hydrogen.
Scientific Research
Helium's unique properties are crucial in various scientific research areas, including nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry, and studies involving superconductivity. Its inertness makes it an ideal carrier gas in many analytical techniques.
Beyond the Basics: Exploring Excited States
While the ground state electron configuration (1s²) is the most stable and common state for helium, it can also exist in excited states when an electron absorbs energy and jumps to a higher energy level. For instance, an excited state could be represented as 1s¹2s¹, indicating one electron in the 1s orbital and one in the 2s orbital. These excited states are transient and eventually decay back to the ground state, emitting photons in the process. This emission of photons is responsible for helium's characteristic spectral lines.
Helium and the Periodic Table: A Noble Gas Perspective
Helium's position in the periodic table, as the first element in Group 18 (noble gases), reflects its unique electron configuration and chemical inertness. The noble gases, including helium, neon, argon, krypton, xenon, and radon, are all characterized by their complete outermost electron shells, leading to exceptional stability and low reactivity.
Conclusion: The Significance of Simplicity
Helium, despite its simplicity with only two electrons, offers a profound illustration of fundamental principles in atomic structure and chemical behavior. Its electron configuration, 1s², is not merely a notation but a key to understanding its inertness, low boiling point, and unique applications. The insights gained from studying helium's electron configuration extend far beyond its individual properties, providing a cornerstone for understanding the behavior of more complex elements and the periodic table as a whole. Further exploration of helium's interactions with light, its behavior under extreme conditions, and its role in astrophysics continue to unveil new facets of this seemingly simple, yet remarkably important, element. Understanding its electron configuration unlocks the door to understanding its crucial role in science and technology.
Latest Posts
Latest Posts
-
Whats The Square Root Of 1 4
Mar 23, 2025
-
3 5 As A Decimal And Percent
Mar 23, 2025
-
When Nacl Is Dissolved In Water
Mar 23, 2025
-
Boiling Of Water Is A Physical Change
Mar 23, 2025
-
What Are The Common Factors Of 24 And 32
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Helium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
