Boiling Of Water Is A Physical Change
listenit
Mar 23, 2025 · 5 min read
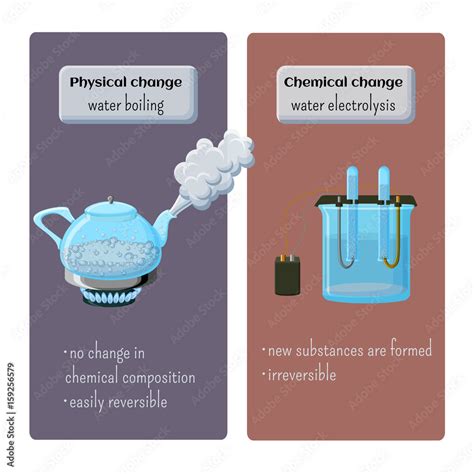
Table of Contents
Boiling Water: A Deep Dive into Physical Change
Boiling water is a quintessential example of a physical change, a transformation that alters the form or appearance of a substance but doesn't change its chemical composition. While seemingly simple, the process of boiling water offers a fascinating window into the world of physics and chemistry, illustrating key concepts like phase transitions, heat transfer, and the properties of matter. This comprehensive exploration will delve deep into the science behind boiling water, solidifying its classification as a physical change and debunking any misconceptions.
Understanding Physical Changes vs. Chemical Changes
Before diving into the specifics of boiling water, let's establish a clear distinction between physical and chemical changes. A physical change affects the physical properties of a substance, like its shape, size, or state of matter (solid, liquid, gas). Crucially, the chemical composition remains unaltered. Examples include melting ice, dissolving sugar in water, or, as we'll extensively explore, boiling water.
A chemical change, on the other hand, alters the chemical composition of a substance. New substances with different properties are formed. This often involves chemical reactions, indicated by signs like color change, gas production, or temperature change. Burning wood, rusting iron, or baking a cake are all examples of chemical changes.
The Science of Boiling Water: A Physical Transformation
Boiling water is a phase transition, specifically a transition from the liquid phase to the gaseous phase (vaporization). This transition occurs when the water reaches its boiling point, which is 100°C (212°F) at standard atmospheric pressure. But what exactly is happening at a molecular level?
Molecular Movement and Heat Energy
Water molecules are constantly in motion, vibrating and colliding with each other. The intensity of this movement is directly related to temperature. As heat energy is added to water, the molecules absorb this energy, increasing their kinetic energy – the energy of motion. This leads to faster and more energetic collisions.
Breaking Intermolecular Forces
Water molecules are held together by intermolecular forces, primarily hydrogen bonds. These bonds are relatively strong compared to other liquids, contributing to water's high boiling point. As the water heats up, the increased kinetic energy of the molecules eventually overcomes these intermolecular forces.
The Formation of Water Vapor
When the kinetic energy of the water molecules surpasses the strength of the hydrogen bonds, molecules escape from the liquid's surface and enter the gaseous phase, forming water vapor or steam. This process happens throughout the liquid's volume as the temperature continues to rise, creating bubbles of steam that rise to the surface.
Boiling Point and Pressure: A Delicate Balance
The boiling point of water is not a fixed constant; it's dependent on atmospheric pressure. At higher altitudes, where the atmospheric pressure is lower, water boils at a lower temperature. Conversely, at higher pressures, the boiling point increases. This is why pressure cookers can cook food faster – the increased pressure raises the boiling point, allowing the water to reach higher temperatures and cook the food more quickly.
Why Boiling Water is Irreversibly Physical
While many physical changes are reversible (e.g., melting ice can be frozen back into ice), the boiling of water appears irreversible at first glance. However, the change itself remains fundamentally physical.
The apparent irreversibility comes from the fact that the water vapor disperses into the atmosphere. To reverse the process, we need to condense the water vapor back into liquid water. This involves cooling the steam, removing heat energy, and allowing the water molecules to slow down and reform hydrogen bonds. This condensation is still a physical change. No new chemical substances are formed; it’s just a change of state.
Debunking Misconceptions: Is It Chemical?
Some might argue that since bubbles are forming, and there is a change of state, it might be a chemical change. However, the bubbles are purely water vapor – H₂O in its gaseous phase. No new chemical compounds are produced. The process of boiling is simply a change in the physical state of the water, driven solely by the addition of heat energy.
The absence of any new chemical product is the key differentiator. If a chemical change had occurred, we would expect to see the formation of new substances with different properties. This does not happen when water boils.
Applications and Real-World Examples
The physical change of boiling water is fundamental to countless processes in our daily lives and across various industries.
Cooking and Food Preparation
Boiling water is essential for cooking many foods, from pasta and vegetables to eggs and grains. The process uses heat transfer to cook the food evenly.
Sanitation and Sterilization
Boiling water is a simple and effective method for sanitizing surfaces and sterilizing tools. The high temperature kills many harmful microorganisms.
Industrial Processes
Boiling water is utilized in numerous industrial applications, including power generation (steam turbines), cleaning and rinsing processes, and chemical reactions (as a solvent or reactant in some cases, though this involvement is often part of a larger chemical process).
Scientific Research
Boiling and the subsequent condensation of water are crucial in various laboratory techniques, such as distillation and purification.
Conclusion: The Physical Reality of Boiling
The boiling of water is unequivocally a physical change. The transformation involves a change in the state of matter, from liquid to gas, driven by the absorption of heat energy and the overcoming of intermolecular forces. While the process may seem irreversible in everyday scenarios due to the dispersion of water vapor, the underlying transformation is purely physical. No new chemical substances are created, and the reverse process, condensation, is also a physical change. Understanding this fundamental physical change is crucial in numerous applications, from daily routines to large-scale industrial processes and scientific research. The seemingly simple act of boiling water offers a powerful illustration of the principles of physics and chemistry at work.
Latest Posts
Latest Posts
-
6 Out Of 11 As A Percentage
Mar 25, 2025
-
How Do Compounds Differ From Elements
Mar 25, 2025
-
What Percent Is 7 Out Of 20
Mar 25, 2025
-
Which Color Of Light Has The Most Energy
Mar 25, 2025
-
Differentiate Between Empirical Formula And Molecular Formula
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Boiling Of Water Is A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
