What Is The Electron Arrangement For Aluminum
listenit
Mar 29, 2025 · 5 min read
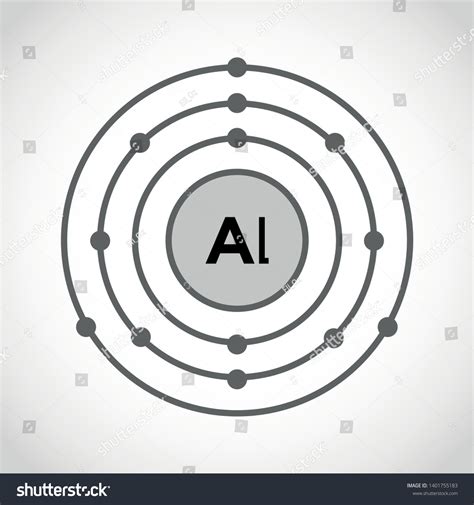
Table of Contents
What is the Electron Arrangement for Aluminum? A Deep Dive into Atomic Structure
Aluminum, a ubiquitous metal found in everything from soda cans to aircraft parts, boasts a fascinating atomic structure. Understanding its electron arrangement is key to comprehending its properties and reactivity. This comprehensive guide delves into the intricacies of aluminum's electron configuration, exploring its implications for chemical bonding, physical characteristics, and its role in various applications.
Understanding Electron Configuration
Before diving into aluminum specifically, let's establish a foundational understanding of electron configuration. An atom's electron configuration describes how electrons are distributed among its various energy levels and sublevels. These energy levels, also known as shells, are regions surrounding the nucleus where electrons are most likely to be found. Each shell has a specific capacity for electrons.
The first shell (n=1) can hold a maximum of two electrons, while the second shell (n=2) can accommodate up to eight. The third shell (n=3) can hold up to 18 electrons, and so on. Within each shell are subshells (s, p, d, f), each with its own characteristic shape and capacity. The 's' subshell can hold a maximum of two electrons, the 'p' subshell six, the 'd' subshell ten, and the 'f' subshell fourteen.
Electron configuration is often represented using a shorthand notation, indicating the principal quantum number (n), the subshell (s, p, d, f), and the number of electrons in that subshell. For example, 1s² represents two electrons in the 1s subshell.
The Electron Arrangement of Aluminum (Al)
Aluminum, with an atomic number of 13, possesses 13 protons in its nucleus and, in its neutral state, 13 electrons surrounding it. Following the Aufbau principle (filling orbitals from lowest to highest energy), the electron configuration of aluminum is:
1s² 2s² 2p⁶ 3s² 3p¹
Let's break this down:
- 1s²: Two electrons occupy the first energy level's 's' subshell.
- 2s²: Two electrons fill the second energy level's 's' subshell.
- 2p⁶: Six electrons complete the second energy level's 'p' subshell.
- 3s²: Two electrons occupy the third energy level's 's' subshell.
- 3p¹: A single electron occupies the third energy level's 'p' subshell.
Orbital Diagrams and Electron Configuration
A more visual representation of aluminum's electron configuration utilizes orbital diagrams. Each orbital within a subshell can hold a maximum of two electrons with opposite spins (represented by arrows pointing up and down). For aluminum:
- 1s: ↑↓
- 2s: ↑↓
- 2p: ↑↓ ↑↓ ↑↓
- 3s: ↑↓
- 3p: ↑ (one electron occupies one of the three 3p orbitals)
This clearly illustrates that aluminum has three valence electrons—electrons in the outermost energy level (n=3). These valence electrons are crucial in determining aluminum's chemical behavior and bonding characteristics.
Aluminum's Reactivity and Chemical Bonding
The presence of three valence electrons dictates aluminum's reactivity. Aluminum readily loses these three valence electrons to achieve a stable octet configuration, resembling the noble gas neon. This electron loss results in the formation of a +3 ion (Al³⁺).
Aluminum's tendency to lose electrons makes it a highly reactive metal, readily participating in various chemical reactions, including:
-
Oxidation: Aluminum readily reacts with oxygen in the air, forming a protective layer of aluminum oxide (Al₂O₃). This oxide layer is incredibly strong and prevents further oxidation, making aluminum remarkably resistant to corrosion. This is a crucial property that contributes to its widespread use in various applications.
-
Reactions with acids and bases: Aluminum reacts with both acids and bases, generating hydrogen gas in the process. This reactivity is utilized in certain industrial processes.
-
Formation of alloys: Aluminum's ability to form alloys with other metals, such as copper, magnesium, and zinc, enhances its strength, durability, and other desirable properties. These alloys find applications in diverse fields, from construction to aerospace engineering.
Aluminum's Physical Properties and Electron Configuration
Aluminum's physical properties are intimately linked to its electron configuration. The relatively weak metallic bonding arising from the three valence electrons contributes to its:
-
Lightweight nature: Aluminum is significantly lighter than many other metals, making it ideal for applications where weight reduction is crucial, such as aircraft construction.
-
High ductility and malleability: The ease with which aluminum atoms can slide past one another allows it to be easily shaped and drawn into wires, contributing to its versatility in manufacturing.
-
Excellent conductivity: The relatively free movement of valence electrons enables aluminum to conduct electricity and heat efficiently. This property is exploited in electrical wiring, heat sinks, and other applications requiring efficient energy transfer.
-
Reflectivity: The interaction of light with the delocalized electrons in aluminum gives it its characteristic reflective properties, making it useful in mirrors and other optical applications.
Aluminum's Applications and Its Electron Configuration
The unique combination of properties stemming from its electron configuration makes aluminum an indispensable element in countless applications:
-
Packaging: Aluminum's corrosion resistance, lightweight nature, and malleability make it ideal for creating beverage cans, food packaging, and other containers.
-
Transportation: Aluminum's lightweight yet strong alloys are widely used in automobiles, aircraft, trains, and ships to improve fuel efficiency and structural integrity.
-
Construction: Aluminum's durability, corrosion resistance, and recyclability make it a popular choice for building materials, including window frames, roofing, and siding.
-
Electrical applications: Aluminum's excellent electrical conductivity makes it a vital component in electrical wiring, power transmission lines, and electronic devices.
Conclusion: The Significance of Aluminum's Electron Configuration
Understanding the electron arrangement of aluminum – 1s² 2s² 2p⁶ 3s² 3p¹ – is pivotal to comprehending its remarkable properties and diverse applications. The presence of three valence electrons dictates its reactivity, its ability to form strong bonds, and its unique physical characteristics. This knowledge allows scientists and engineers to harness aluminum's potential, leading to its widespread use in a vast array of technologies impacting our daily lives. The simple yet profound arrangement of 13 electrons within the aluminum atom underscores the fundamental role of atomic structure in determining the properties of matter. Future research and innovation will undoubtedly continue to explore and exploit the full potential of this versatile metal, further solidifying its place as a cornerstone material of modern civilization. The insights gained from understanding aluminum's electron configuration serve as a microcosm of the broader study of atomic structure and its implications for materials science and engineering. This knowledge empowers us to design, develop, and utilize materials with specific properties, advancing technology and improving quality of life.
Latest Posts
Latest Posts
-
What Type Of Rock Is Most Fossils Found In
Mar 31, 2025
-
Do Lone Pairs Count As Sigma Bonds
Mar 31, 2025
-
What Is One Third In Decimal
Mar 31, 2025
-
Area Of A Circle 9 Inch Diameter
Mar 31, 2025
-
The Lock And Key Mechanism Refers To
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Arrangement For Aluminum . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
