Do Lone Pairs Count As Sigma Bonds
listenit
Mar 31, 2025 · 6 min read
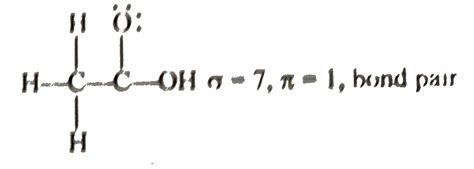
Table of Contents
Do Lone Pairs Count as Sigma Bonds? A Deep Dive into Chemical Bonding
The question of whether lone pairs count as sigma bonds is a common point of confusion for students of chemistry. The short answer is no, lone pairs do not count as sigma bonds. However, understanding why requires a deeper exploration of chemical bonding theory. This article will delve into the fundamental concepts of sigma bonds, lone pairs, and molecular orbital theory to clarify this crucial distinction.
Understanding Sigma Bonds
A sigma (σ) bond is the strongest type of covalent bond formed by the head-on overlap of atomic orbitals. This head-on overlap results in a high electron density concentrated along the internuclear axis connecting the two bonded atoms. Sigma bonds are crucial for the stability and structure of most molecules. They are typically formed by the overlap of:
- s orbitals: An s orbital is spherically symmetrical, and the overlap of two s orbitals forms a single sigma bond.
- s and p orbitals: An s orbital can overlap with a p orbital along the p orbital's axis to form a sigma bond.
- p orbitals: Two p orbitals can overlap head-on to form a sigma bond.
Key Characteristics of Sigma Bonds:
- Head-on overlap: This is the defining characteristic of a sigma bond.
- Free rotation: Atoms connected by a sigma bond can freely rotate around the bond axis.
- Strong bond strength: Sigma bonds are generally stronger than pi bonds.
- Single bond: A single bond between two atoms is always a sigma bond.
Understanding Lone Pairs
Lone pairs, also known as non-bonding pairs, are pairs of electrons that are not involved in bonding. They are localized on a single atom and contribute to the atom's electron configuration and overall shape of the molecule. These electrons occupy atomic orbitals and influence the molecule's geometry through their repulsive interactions with bonding electrons. Lone pairs significantly affect:
- Molecular geometry: Lone pairs occupy space and exert repulsive forces on bonding pairs, influencing the bond angles and overall shape of the molecule (VSEPR theory).
- Polarity: Lone pairs contribute to the overall electron distribution in a molecule, leading to dipole moments and polarity.
- Reactivity: Lone pairs can participate in chemical reactions as Lewis bases, donating their electron density to electron-deficient species.
The Crucial Difference: Shared vs. Unshared Electrons
The fundamental difference between a sigma bond and a lone pair lies in the nature of the electrons. A sigma bond involves a shared pair of electrons between two atoms, holding them together. A lone pair, however, consists of unshared electrons that belong exclusively to a single atom. While both involve electron pairs, their role in molecular structure and bonding is distinctly different. The electrons in a sigma bond are delocalized across the bonding atoms, contributing directly to the bond strength and stability. Lone pair electrons are localized on a single atom, contributing to the atom's overall electronic structure but not directly participating in the bonding between atoms.
Molecular Orbital Theory and Lone Pairs
Molecular orbital (MO) theory provides a more sophisticated understanding of chemical bonding. In MO theory, atomic orbitals combine to form molecular orbitals that encompass the entire molecule. Sigma bonding orbitals are formed by the constructive interference of atomic orbitals, resulting in a stable bonding molecular orbital with increased electron density between the nuclei.
Lone pairs in MO theory are represented by non-bonding molecular orbitals. These molecular orbitals are localized on a single atom and are not involved in the bonding between atoms. They are formed from atomic orbitals that do not participate in the formation of bonding orbitals. These non-bonding orbitals are filled with electron pairs that contribute to the overall electron density of the molecule but do not directly contribute to bonding.
Why Lone Pairs Aren't Sigma Bonds
The key reason lone pairs are not considered sigma bonds is their lack of involvement in the direct bonding between atoms. Sigma bonds are fundamentally about the sharing of electrons between two atoms to form a stable bond. Lone pairs, despite being electron pairs, are not shared between atoms. Their presence influences molecular geometry and properties, but they do not directly contribute to the formation of a bond between two nuclei.
Think of it this way: a sigma bond is like a bridge connecting two islands (atoms). The bridge (sigma bond) requires materials (shared electrons) to be built and holds the islands together. A lone pair, on the other hand, is like a pile of materials (unshared electrons) on a single island. These materials are important for the island's characteristics, but they don't create a connection to another island.
Examples Illustrating the Distinction
Let's consider a few examples to reinforce this concept:
1. Water (H₂O): Oxygen has two lone pairs and two bonding pairs. The two bonding pairs form two sigma bonds with the hydrogen atoms. The lone pairs do not contribute to the O-H bonds.
2. Ammonia (NH₃): Nitrogen has one lone pair and three bonding pairs. The three bonding pairs form three sigma bonds with the hydrogen atoms. The lone pair influences the shape (trigonal pyramidal) but doesn't directly participate in the N-H bonds.
3. Methane (CH₄): Carbon has four bonding pairs, forming four sigma bonds with the hydrogen atoms. There are no lone pairs in methane.
In all these examples, the lone pairs contribute to the overall molecular geometry and reactivity but are distinct from the sigma bonds that hold the atoms together.
Beyond Sigma Bonds: Pi Bonds and Hybrid Orbitals
It's important to note that while sigma bonds are fundamental to most covalent molecules, other types of bonds exist. Pi (π) bonds, for example, are formed by the sideways overlap of p orbitals, resulting in electron density above and below the internuclear axis. Pi bonds are generally weaker than sigma bonds.
Additionally, the concept of hybrid orbitals helps explain the bonding in molecules with complex geometries. Hybrid orbitals are formed by the mixing of atomic orbitals to produce new orbitals with different shapes and energies. These hybrid orbitals often participate in the formation of sigma bonds. However, the presence of hybrid orbitals does not change the fundamental distinction between lone pairs and sigma bonds. Lone pairs occupy non-bonding orbitals, regardless of whether the bonding orbitals involved are hybrid or non-hybrid orbitals.
Conclusion: Lone Pairs' Impact, Though Not Bonds
In summary, while lone pairs are crucial for determining a molecule's shape, polarity, and reactivity, they do not count as sigma bonds. The key difference lies in the sharing of electrons. Sigma bonds involve shared electrons directly participating in bonding between two atoms; lone pairs are unshared electron pairs localized on a single atom. Understanding this distinction is fundamental to mastering the complexities of chemical bonding and molecular structure. This detailed explanation, encompassing sigma bonds, lone pairs, molecular orbital theory, and illustrative examples, should provide a comprehensive understanding of why lone pairs are not considered sigma bonds. This knowledge is essential for accurately predicting molecular properties and understanding chemical reactions. Remember, the presence and positioning of lone pairs are critically important for understanding a molecule's behavior, even though they are not sigma bonds themselves.
Latest Posts
Latest Posts
-
What Is The Lcm For 6 And 10
Apr 02, 2025
-
Which Organelles Supply Energy To The Cell
Apr 02, 2025
-
Why Is Density A Physical Property
Apr 02, 2025
-
Is Koh A Base Or Acid
Apr 02, 2025
-
Why Did Small States Object To The Virginia Plan
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Do Lone Pairs Count As Sigma Bonds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
