What Is The Difference Between Monomers And Polymers
listenit
Mar 24, 2025 · 6 min read
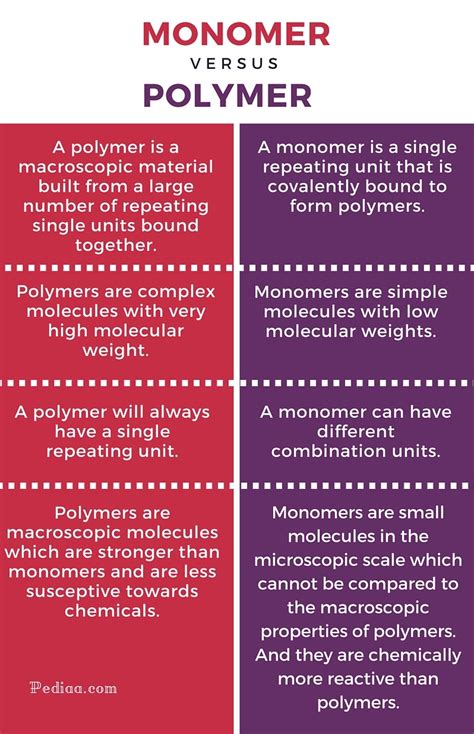
Table of Contents
What's the Difference Between Monomers and Polymers? A Deep Dive
Understanding the difference between monomers and polymers is fundamental to grasping many aspects of chemistry and materials science. These terms are frequently encountered in discussions about plastics, proteins, DNA, and a vast array of other materials crucial to our daily lives. While seemingly simple, the distinctions between these building blocks and the resulting structures are rich and multifaceted. This article will delve deep into the differences, exploring their chemical properties, diverse applications, and the processes that link them.
Defining Monomers: The Basic Building Blocks
A monomer is a small molecule that can react with other monomers to form a larger molecule known as a polymer. Think of monomers as individual LEGO bricks—small, simple units that can be combined to create complex structures. Critically, monomers possess reactive functional groups, which are specific atoms or groups of atoms within the molecule that are capable of forming chemical bonds with other monomers. These functional groups are the key to polymerization, the process by which monomers link together. Common functional groups include hydroxyl (-OH), carboxyl (-COOH), amino (-NH2), and alkene (C=C) groups. The type of functional group largely dictates the type of polymerization reaction that occurs.
Examples of Monomers:
- Ethylene (CH2=CH2): A simple alkene that serves as the monomer for polyethylene, one of the most common plastics.
- Styrene (C8H8): Used to produce polystyrene, another widely used plastic found in packaging and insulation.
- Amino acids: These molecules contain both amino (-NH2) and carboxyl (-COOH) groups. They are the monomers of proteins, essential biological macromolecules.
- Nucleotides: These complex molecules are the monomers of nucleic acids like DNA and RNA, the carriers of genetic information.
- Glucose (C6H12O6): Although often considered a single molecule, glucose acts as a monomer in the formation of starch and cellulose, vital polysaccharides in plants.
The specific structure and properties of a monomer directly influence the properties of the resulting polymer. For instance, the presence of bulky side groups on a monomer can affect the flexibility and strength of the resulting polymer chain.
Defining Polymers: The Macromolecular Result
A polymer is a large molecule composed of many repeating smaller units, called monomers, linked together chemically. Returning to the LEGO analogy, the polymer would be the complex structure built from many individual bricks. These long chains can be linear, branched, or cross-linked, leading to significant variations in material properties. The term "macromolecule" emphasizes the large size of polymers, often containing thousands or even millions of monomer units.
Types of Polymers:
The diversity of polymers stems from several factors:
- Monomer type: Different monomers yield different polymers with unique characteristics.
- Chain length: Longer chains generally lead to stronger and more rigid materials.
- Branching: Branched polymers tend to be less dense and have lower melting points than linear polymers.
- Cross-linking: Cross-links between polymer chains enhance strength and rigidity.
Classification based on origin: Polymers are also classified as either natural or synthetic:
- Natural polymers: These polymers occur naturally in living organisms. Examples include proteins (made of amino acids), nucleic acids (DNA and RNA, made of nucleotides), cellulose (made of glucose), and rubber (made of isoprene).
- Synthetic polymers: These are polymers produced industrially. Familiar examples include polyethylene (plastic bags), polystyrene (foam cups), nylon (clothing fibers), and Teflon (non-stick coatings).
Properties of Polymers:
The properties of polymers vary considerably depending on their structure and composition. Some common properties include:
- Flexibility: Some polymers, like polyethylene, are flexible, while others, like Bakelite, are rigid.
- Strength: The strength of a polymer can range from extremely strong (like Kevlar) to relatively weak.
- Thermal stability: Some polymers withstand high temperatures, while others soften or melt at relatively low temperatures.
- Solubility: Solubility in various solvents differs greatly depending on the polymer's structure and polarity.
- Elasticity: Certain polymers exhibit elasticity, meaning they can stretch and return to their original shape.
The Polymerization Process: Joining Monomers
The process of converting monomers into polymers is called polymerization. There are several types of polymerization mechanisms, each with its own unique characteristics:
Addition Polymerization:
In addition polymerization, monomers add to each other without the loss of any atoms. This usually occurs through the opening of a double bond (like in alkenes) or a ring structure. The reaction proceeds through a chain reaction mechanism involving initiation, propagation, and termination steps. Examples of polymers formed via addition polymerization include polyethylene, polypropylene, and polystyrene.
Condensation Polymerization:
In condensation polymerization, monomers combine by eliminating a small molecule, often water. This process typically involves monomers with two or more functional groups capable of reacting with each other. Examples include nylon (formed from diamines and diacids) and polyester (formed from diols and diacids).
Other Polymerization Techniques:
Beyond addition and condensation, other techniques exist, including ring-opening polymerization, living polymerization, and enzymatic polymerization. These specialized methods allow for greater control over polymer architecture and properties.
Applications of Monomers and Polymers: A Vast Landscape
The applications of monomers and polymers span virtually every aspect of modern life. This is due to the incredible diversity in their properties and the ability to tailor these properties through careful selection of monomers and polymerization techniques.
Polymers in Everyday Life:
- Packaging: Polyethylene, polypropylene, and polystyrene are ubiquitous in packaging materials, ranging from plastic bags to food containers.
- Textiles: Nylon, polyester, and acrylic fibers are commonly used in clothing and other textiles.
- Construction: Polyvinyl chloride (PVC) is used in pipes and other construction materials.
- Automotive industry: Polymers are used extensively in car parts, including bumpers, dashboards, and interior components.
- Medical applications: Polymers are used in medical devices, implants, and drug delivery systems.
Monomers in Biological Systems:
Monomers are the fundamental building blocks of life.
- Proteins: Amino acids are the monomers of proteins, which perform a vast array of functions in living organisms, including catalysis, structural support, and transport.
- Nucleic Acids: Nucleotides are the monomers of DNA and RNA, which store and transmit genetic information.
- Carbohydrates: Sugars, such as glucose, act as monomers for polysaccharides like starch and cellulose, providing energy storage and structural support in plants.
Future Trends in Monomer and Polymer Research:
Research continues to push the boundaries of monomer and polymer science. Key areas of focus include:
- Sustainable polymers: Developing polymers from renewable resources and that are biodegradable or recyclable is crucial for environmental sustainability.
- Bio-inspired polymers: Learning from nature to design polymers with enhanced properties, such as self-healing capabilities.
- Advanced polymer architectures: Creating polymers with precisely controlled structures to achieve specific functions and properties.
- Polymer nanocomposites: Combining polymers with nanoparticles to create materials with enhanced mechanical, thermal, and electrical properties.
Conclusion: A Dynamic Field with Endless Possibilities
The difference between monomers and polymers lies in their size and complexity. Monomers are the small, reactive building blocks, while polymers are the large macromolecules formed by linking many monomers together. The properties of polymers are highly dependent on the type of monomer, the polymerization method, and the resulting chain structure. This remarkable versatility has led to a vast array of applications across various industries, from packaging to medicine to advanced technologies. The field of polymer science continues to evolve, promising exciting new materials and applications in the years to come. Understanding the fundamental distinction between monomers and polymers is key to appreciating the innovative potential of this dynamic and crucial area of chemistry.
Latest Posts
Latest Posts
-
Common Factors Of 16 And 30
Mar 28, 2025
-
Which Of The Following Is A Transition Element
Mar 28, 2025
-
Do Gases Take The Shape Of Their Container
Mar 28, 2025
-
What Is The Sum Of 667 And 23
Mar 28, 2025
-
What Is The Conjugate Acid For Hco3
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Monomers And Polymers . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
