Do Gases Take The Shape Of Their Container
listenit
Mar 28, 2025 · 6 min read
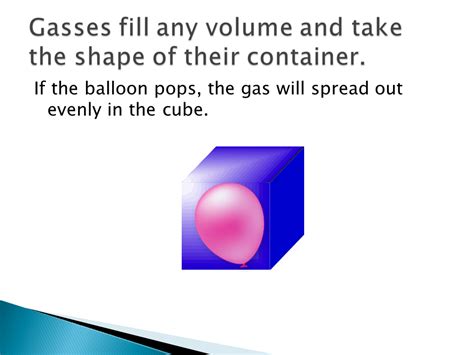
Table of Contents
Do Gases Take the Shape of Their Container? A Deep Dive into Gas Properties
Gases are all around us, forming the air we breathe, the carbon dioxide we exhale, and even the helium in party balloons. But what exactly is a gas, and why do they exhibit such unique properties, like taking the shape of their container? This comprehensive article will delve into the microscopic world of gases to explain this fundamental characteristic and explore related concepts. We'll examine the kinetic molecular theory, gas laws, and real-world applications to provide a thorough understanding of this fascinating aspect of matter.
Understanding the Kinetic Molecular Theory of Gases
The behavior of gases, including their ability to conform to the shape and volume of their container, is best explained by the kinetic molecular theory (KMT). This theory posits several key assumptions about the nature of gases:
Key Assumptions of the Kinetic Molecular Theory:
- Gases are composed of tiny particles: These particles are usually atoms or molecules, and their size is negligible compared to the distances between them. This means that the volume occupied by the gas particles themselves is insignificant compared to the total volume of the gas.
- Gas particles are in constant, random motion: They move in straight lines until they collide with each other or the walls of the container. These collisions are elastic, meaning no kinetic energy is lost during the collision.
- Gas particles have no attractive or repulsive forces between them: This implies that the particles interact very little with each other, except during collisions. This assumption is more accurate for ideal gases, and real gases exhibit some intermolecular forces.
- The average kinetic energy of gas particles is directly proportional to the absolute temperature: This means that as the temperature increases, the particles move faster, and vice-versa. This relationship is a cornerstone of understanding gas behavior.
Why Gases Take the Shape of Their Container: A Microscopic Perspective
The constant, random motion of gas particles is the key to understanding why they adopt the shape of their container. Imagine a gas confined within a container:
- No fixed positions: Unlike solids, gas particles do not occupy fixed positions in a lattice structure. They are free to move throughout the entire volume of the container.
- Collisions with container walls: As the gas particles move randomly, they constantly collide with the walls of the container. These collisions exert pressure on the walls.
- Shape conformity: Because the particles are free to move and occupy any space within the container, they distribute themselves evenly, completely filling the available volume. This results in the gas taking on the exact shape of the container, regardless of its form—be it a spherical balloon, a rectangular box, or an oddly shaped flask.
Ideal Gases vs. Real Gases: Deviations from the Ideal Model
The kinetic molecular theory describes the behavior of ideal gases, a theoretical model that perfectly adheres to the KMT assumptions. However, real gases deviate from ideal behavior, especially under conditions of high pressure and low temperature.
Factors Affecting Real Gas Behavior:
- Intermolecular forces: Real gas molecules do exhibit attractive forces (like van der Waals forces) between them, especially at lower temperatures when the particles are moving slower. These forces can cause the gas to deviate from the ideal gas law.
- Molecular volume: The volume of real gas molecules is not negligible compared to the total volume of the gas, particularly at high pressures where the molecules are closer together.
Gas Laws: Mathematical Descriptions of Gas Behavior
Several gas laws mathematically describe the relationship between pressure (P), volume (V), temperature (T), and the amount of gas (n, often expressed in moles). These laws are excellent approximations for ideal gases and provide a useful framework for understanding gas behavior.
Boyle's Law: The Relationship Between Pressure and Volume
Boyle's Law states that at constant temperature, the pressure of a gas is inversely proportional to its volume. This means that as the pressure increases, the volume decreases, and vice-versa. This is easily visualized by thinking of a balloon—squeezing it (increasing pressure) reduces its volume. Mathematically, it's expressed as: P₁V₁ = P₂V₂
Charles's Law: The Relationship Between Volume and Temperature
Charles's Law states that at constant pressure, the volume of a gas is directly proportional to its absolute temperature (in Kelvin). As the temperature increases, the volume increases, and vice-versa. Think of a hot air balloon—the heated air expands, increasing the balloon's volume. The mathematical representation is: V₁/T₁ = V₂/T₂
Gay-Lussac's Law: The Relationship Between Pressure and Temperature
Gay-Lussac's Law states that at constant volume, the pressure of a gas is directly proportional to its absolute temperature. As the temperature increases, the pressure increases, and vice-versa. This is why pressure cookers build up pressure as they heat up. The equation is: P₁/T₁ = P₂/T₂
Avogadro's Law: The Relationship Between Volume and Amount of Gas
Avogadro's Law states that at constant temperature and pressure, the volume of a gas is directly proportional to the number of moles of gas. This means that doubling the amount of gas (at constant T and P) doubles the volume. The equation is: V₁/n₁ = V₂/n₂
The Ideal Gas Law: Combining All the Relationships
The ideal gas law combines all the above laws into a single equation: PV = nRT, where R is the ideal gas constant. This equation is extremely useful for calculating any of the four variables (P, V, n, T) if the other three are known.
Real-World Applications: How Understanding Gas Behavior Impacts Our Lives
The understanding of how gases behave has numerous practical applications in our daily lives and various industries:
- Weather forecasting: Meteorologists utilize gas laws to predict weather patterns, considering changes in temperature, pressure, and humidity (the amount of water vapor in the air).
- Aerosol cans: These cans rely on the pressure exerted by compressed gases to dispense their contents. Understanding the relationship between pressure and temperature is crucial in their design and safe operation.
- Breathing and respiration: Our lungs expand and contract, changing the volume and pressure within them, to facilitate the inhalation and exhalation of gases.
- Automobile engines: Internal combustion engines rely on the controlled expansion of gases produced by burning fuel to generate power.
- Industrial processes: Many industrial processes, such as the production of ammonia (Haber-Bosch process) and the refining of petroleum, involve manipulating the conditions (temperature, pressure) of gases to control reactions and yields.
- Diving and aviation: Divers and pilots must account for changes in air pressure at different altitudes to avoid health risks like decompression sickness (the bends).
Conclusion: The Ever-Expanding World of Gas Behavior
The ability of gases to conform to the shape of their container is a direct consequence of the kinetic molecular theory, specifically the constant, random motion and negligible volume of gas particles. While the ideal gas law provides a simplified model, understanding the deviations exhibited by real gases under various conditions is crucial for accurate predictions and practical applications. From weather forecasting to industrial processes, the principles governing gas behavior are fundamental to numerous fields, highlighting the importance of this fundamental concept in chemistry and beyond. Further exploration into advanced topics like statistical mechanics and thermodynamics can provide even deeper insights into the fascinating world of gases.
Latest Posts
Latest Posts
-
How Many Pints Are In 1 Pound
Mar 31, 2025
-
How Much Is Half A Gallon In Cups
Mar 31, 2025
-
What Is 5 5 As A Decimal
Mar 31, 2025
-
Is Gasoline Evaporating A Chemical Change
Mar 31, 2025
-
What Is Half Of A Mile In Feet
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Do Gases Take The Shape Of Their Container . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
