What Is The Conjugate Acid For Hco3-
listenit
Mar 28, 2025 · 5 min read
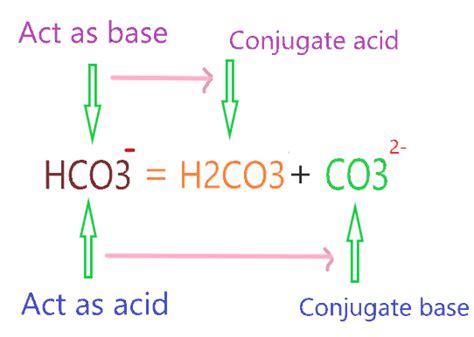
Table of Contents
What is the Conjugate Acid for HCO₃⁻? A Deep Dive into Acid-Base Chemistry
Understanding conjugate acid-base pairs is fundamental to grasping acid-base chemistry. This article delves deep into the concept, focusing specifically on the conjugate acid of the bicarbonate ion, HCO₃⁻. We'll explore the definition of conjugate acid-base pairs, the Brønsted-Lowry theory, the properties of HCO₃⁻, and finally, definitively answer the question: what is the conjugate acid of HCO₃⁻? We'll also explore some related concepts and applications.
Understanding Conjugate Acid-Base Pairs
According to the Brønsted-Lowry theory, an acid is a proton (H⁺) donor, and a base is a proton acceptor. A conjugate acid-base pair consists of two species that differ by a single proton (H⁺). When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid.
Think of it like this: an acid loses a proton to become its conjugate base, and a base gains a proton to become its conjugate acid. They are chemically related, differing only by the presence or absence of a single proton.
The Bicarbonate Ion (HCO₃⁻): A Versatile Species
The bicarbonate ion, HCO₃⁻, is an amphiprotic species, meaning it can act as both an acid and a base. This dual nature stems from its ability to either donate or accept a proton.
- As an acid: HCO₃⁻ can donate a proton (H⁺), forming the carbonate ion (CO₃²⁻).
- As a base: HCO₃⁻ can accept a proton (H⁺), forming carbonic acid (H₂CO₃).
This amphiprotic behavior is crucial in understanding its role in various biological and chemical systems, including blood buffering and the carbon cycle.
Identifying the Conjugate Acid of HCO₃⁻
Given that HCO₃⁻ can act as a base, accepting a proton, its conjugate acid is formed when it gains that proton. Therefore, the conjugate acid of HCO₃⁻ is carbonic acid (H₂CO₃).
HCO₃⁻ + H⁺ ⇌ H₂CO₃
This equilibrium reaction shows the interconversion between the bicarbonate ion and its conjugate acid, carbonic acid. The reaction is reversible, meaning carbonic acid can also donate a proton to reform the bicarbonate ion.
Properties of Carbonic Acid (H₂CO₃)
Carbonic acid is a weak acid, meaning it only partially dissociates in water. It's relatively unstable and readily decomposes into carbon dioxide (CO₂) and water (H₂O):
H₂CO₃ ⇌ H₂O + CO₂
This decomposition is important in several biological processes and in the carbon cycle. The equilibrium between carbonic acid, bicarbonate, and carbonate ions is crucial for maintaining the pH balance in various systems, notably blood.
The Importance of Conjugate Acid-Base Pairs in Buffer Systems
The HCO₃⁻/H₂CO₃ conjugate pair plays a vital role in maintaining the pH of blood. This system acts as a buffer, resisting changes in pH when small amounts of acid or base are added. Buffers are crucial for maintaining the stability of biological systems, as significant pH fluctuations can be detrimental.
When an acid is added to the blood, the bicarbonate ion (HCO₃⁻) acts as a base, accepting the protons and forming carbonic acid (H₂CO₃). This minimizes the change in pH. Conversely, when a base is added, carbonic acid (H₂CO₃) acts as an acid, donating protons to neutralize the base and again minimize the pH change.
HCO₃⁻ in Biological Systems: Beyond Blood Buffering
The bicarbonate ion's importance extends beyond blood buffering. It's involved in:
- Carbon dioxide transport: The majority of carbon dioxide produced in cellular respiration is transported in the blood as bicarbonate ions.
- Cellular processes: Bicarbonate ions participate in various metabolic pathways.
- Mineral balance: It plays a role in maintaining the balance of minerals in the body.
Applications of the HCO₃⁻/H₂CO₃ System
The understanding of the HCO₃⁻/H₂CO₃ system has several practical applications:
- Medicine: Intravenous solutions often contain bicarbonate to manage acidosis (increased acidity in the blood).
- Environmental science: The carbonate system is critical in understanding ocean acidification.
- Chemical engineering: The bicarbonate system is used in various chemical processes, such as pH control and buffering in industrial reactions.
Beyond the Basics: A Deeper Look at Equilibrium
The equilibrium between HCO₃⁻ and H₂CO₃ is governed by the acid dissociation constant (Ka) for carbonic acid. This constant reflects the extent to which carbonic acid dissociates into bicarbonate and protons. The Ka value helps in quantitatively describing the acid-base behavior of the system and predicting the pH under different conditions.
Further Exploration: Polyprotic Acids and Bases
Carbonic acid is a diprotic acid, meaning it can donate two protons. This leads to a more complex equilibrium system involving bicarbonate (HCO₃⁻) and carbonate (CO₃²⁻) ions. Understanding polyprotic acid systems requires a grasp of multiple equilibrium constants and their interplay.
Conclusion: The Significance of Conjugate Acid-Base Pairs
The concept of conjugate acid-base pairs is central to understanding acid-base chemistry. The bicarbonate ion (HCO₃⁻) and its conjugate acid, carbonic acid (H₂CO₃), serve as a prime example of this concept's importance, particularly in biological systems. Their roles in blood buffering, carbon dioxide transport, and various other processes underscore the significance of this specific conjugate pair. Furthermore, understanding the equilibrium dynamics and the broader implications of polyprotic acid systems provides a deeper appreciation for the intricate chemistry governing life and the environment. The HCO₃⁻/H₂CO₃ system is a crucial building block in many chemical and biological phenomena, emphasizing the fundamental importance of mastering the principles of conjugate acid-base pairs. By thoroughly understanding this relationship, we can gain a more comprehensive understanding of various chemical and biological processes.
Latest Posts
Latest Posts
-
What Percentage Is 4 Out Of 20
Mar 31, 2025
-
The Mercalli Scale Is A Scale From
Mar 31, 2025
-
Is Iodine A Metal Metalloid Or Nonmetal
Mar 31, 2025
-
Ionic Compound For Calcium And Sulfur
Mar 31, 2025
-
What Is 95 In Fraction Form
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Acid For Hco3- . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
