What Is The Atomic Number Of An Atom Equivalent To
listenit
Mar 30, 2025 · 7 min read
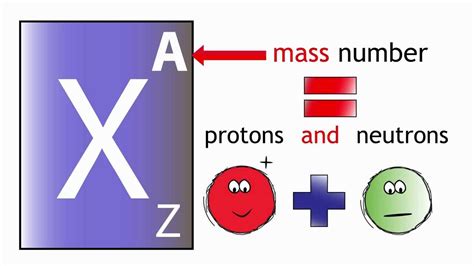
Table of Contents
What is the Atomic Number of an Atom Equivalent To?
The atomic number of an atom is a fundamental property that defines its identity and chemical behavior. It's not simply a number; it represents a crucial aspect of the atom's structure and dictates how it interacts with other atoms. Understanding what the atomic number is equivalent to requires delving into the heart of atomic structure and the nature of matter itself. This comprehensive guide will explore this concept in detail, covering its relationship to protons, electrons, isotopes, the periodic table, and the implications for chemical reactions and properties.
The Atomic Number: A Definition
The atomic number of an atom is equivalent to the number of protons found in its nucleus. The nucleus, a tiny, dense region at the center of the atom, contains the bulk of the atom's mass. This nucleus is composed of two types of particles: protons, which carry a positive electrical charge, and neutrons, which are electrically neutral.
Protons, with their positive charge, play a vital role in determining the atom's overall charge and its interactions with other atoms. The number of protons is absolutely unique to each element. This means that all atoms of a specific element will always have the same number of protons. This is the fundamental defining characteristic that distinguishes one element from another.
Why Protons, Not Electrons or Neutrons?
While the atom also contains electrons (negatively charged particles orbiting the nucleus) and neutrons (neutral particles within the nucleus), it's the number of protons that unequivocally defines the element.
-
Electrons: Atoms can gain or lose electrons, forming ions with different charges. This process, ionization, doesn't change the atom's identity as an element; it simply alters its charge. The number of electrons can vary, but the number of protons remains constant.
-
Neutrons: The number of neutrons can also vary within atoms of the same element, resulting in isotopes. Isotopes have the same number of protons (and thus the same atomic number) but different numbers of neutrons. Although isotopes have slightly different properties, they are still considered the same element because they have the same number of protons.
The Atomic Number and the Periodic Table
The periodic table of elements is organized according to atomic number. Elements are arranged in order of increasing atomic number, which reflects the increasing number of protons in their nuclei. This arrangement isn't arbitrary; it's a testament to the importance of the atomic number in determining an element's properties. Elements with similar chemical properties are grouped together in columns (groups or families), highlighting the relationships between their electron configurations which are directly influenced by the number of protons.
The periodic table is a powerful tool for understanding the relationships between different elements and predicting their behavior based on their position and atomic number. It allows for quick access to information such as the element's symbol, atomic mass, and other important properties directly linked to its atomic structure and number of protons.
Isotopes and Atomic Number
As mentioned earlier, isotopes are atoms of the same element with the same atomic number (same number of protons) but a different number of neutrons. This variation in neutron number affects the atom's mass but not its chemical behavior. For example, carbon-12 (¹²C) and carbon-14 (¹⁴C) are both isotopes of carbon. Both have an atomic number of 6 (six protons), but ¹²C has six neutrons, while ¹⁴C has eight neutrons. The difference in neutron number affects their stability (¹⁴C is radioactive, while ¹²C is stable), but both are still chemically carbon.
The atomic mass listed on the periodic table is a weighted average of the masses of all naturally occurring isotopes of an element. It reflects the abundance of each isotope. Therefore, while the atomic number precisely defines the element, the atomic mass provides an average mass based on the isotopic distribution.
Atomic Number and Chemical Properties
The atomic number is crucial in determining an element's chemical properties. The number of protons directly influences the number of electrons in a neutral atom. These electrons are arranged in energy levels or shells around the nucleus, and the outermost electrons, called valence electrons, are primarily responsible for an element's reactivity and the types of chemical bonds it forms.
The arrangement of these valence electrons determines how an atom will interact with other atoms. Atoms tend to react in ways that achieve a stable electron configuration, often by gaining, losing, or sharing electrons. The number of valence electrons, in turn, is dictated by the atom's overall electron configuration which is fundamentally determined by its atomic number (and therefore the number of protons).
Elements in the same group on the periodic table have similar valence electron configurations, leading to similar chemical behaviors. For example, the alkali metals (Group 1) all have one valence electron, making them highly reactive and readily forming +1 ions. Understanding the relationship between atomic number, electron configuration, and valence electrons is key to predicting chemical reactivity and bond formation.
Atomic Number and Nuclear Reactions
While the atomic number defines the element, it's important to note that nuclear reactions can alter the atomic number. Nuclear reactions involve changes in the nucleus of an atom, unlike chemical reactions which involve changes in the arrangement of electrons only. Processes such as radioactive decay, nuclear fission, and nuclear fusion can change the number of protons in the nucleus, thereby changing the atomic number and transforming one element into another.
For example, in radioactive decay (alpha decay), an atom loses two protons and two neutrons from its nucleus. This results in a decrease in the atomic number by two, effectively transmuting the original element into a different element. Nuclear fission and fusion also involve changes in the atomic number, creating entirely new elements in the process. These reactions are far more energetic than chemical reactions and demonstrate the profound significance of the number of protons in defining an element's fundamental identity.
Practical Applications of Understanding Atomic Number
Understanding the significance of the atomic number has far-reaching implications in various fields:
-
Chemistry: Predicting chemical reactions, understanding bonding, and analyzing chemical properties all rely on knowledge of atomic numbers and electron configurations.
-
Nuclear Physics: Nuclear reactions and the study of radioactivity hinge on the understanding of atomic numbers and their changes during nuclear processes.
-
Material Science: The properties of materials are directly linked to the atomic numbers of the constituent elements. Knowing the atomic number allows for the design and development of materials with specific desired properties.
-
Medicine: Radioactive isotopes, often characterized by their atomic numbers and decay characteristics, are used in medical imaging and treatment.
-
Astronomy: Analyzing the light emitted by stars and other celestial objects allows astronomers to determine the composition of these objects by identifying the elements present based on their characteristic spectral lines and corresponding atomic numbers.
Conclusion: The Defining Identity of an Atom
In conclusion, the atomic number of an atom is far more than just a numerical value. It is equivalent to the number of protons in the atom's nucleus, and this fundamental property defines the element's identity, chemical behavior, and its position within the periodic table. It dictates the number of electrons, the arrangement of electrons within energy levels, the number of valence electrons, and ultimately the chemical properties and reactivity of the element. While isotopes exist with varying numbers of neutrons, the constancy of the proton number (and thus the atomic number) ensures the consistency of the element's identity. Understanding the atomic number is crucial for various scientific disciplines and plays a vital role in numerous applications, ranging from chemical reactions to nuclear physics and beyond. The atomic number serves as the bedrock of our understanding of the elements and the matter that comprises our universe.
Latest Posts
Latest Posts
-
What Is 4 5 In A Fraction
Apr 01, 2025
-
Which Element Is Found In All Organic Compounds
Apr 01, 2025
-
Is Supporting Combustion A Physical Property
Apr 01, 2025
-
100 Cm Equals How Many Meters
Apr 01, 2025
-
Write The Equilibrium Constant Expression For This Reaction
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Atomic Number Of An Atom Equivalent To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
