What Is Standard Temperature In Kelvin
listenit
Mar 30, 2025 · 6 min read
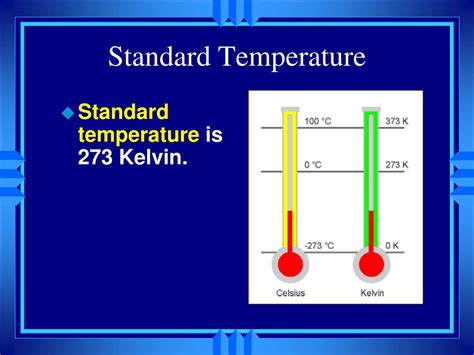
Table of Contents
What is Standard Temperature in Kelvin? A Deep Dive into the Absolute Scale
The concept of "standard temperature" might seem straightforward, but it's actually quite nuanced, especially when considering the Kelvin scale. While there isn't one universally agreed-upon "standard" temperature in Kelvin for all applications, the most commonly used reference points revolve around 273.15 K (0°C, 32°F) and 293.15 K (20°C, 68°F). Understanding these values, and why they're significant, requires delving into the very nature of temperature and the Kelvin scale itself.
Understanding Temperature and its Scales
Temperature, fundamentally, is a measure of the average kinetic energy of the particles within a substance. The higher the kinetic energy, the higher the temperature. Different temperature scales have emerged over time, each with its own zero point and scaling. The most familiar are:
-
Celsius (°C): Based on the freezing and boiling points of water at standard atmospheric pressure (0°C and 100°C respectively). Widely used globally for everyday applications.
-
Fahrenheit (°F): Another common scale, predominantly used in the United States. Its zero point and scaling are based on a different historical convention.
-
Kelvin (K): This is the absolute temperature scale. It's crucial for scientific applications and understanding thermodynamic processes.
The Significance of the Kelvin Scale
The Kelvin scale's defining feature is its absolute zero point. Absolute zero (0 K) represents the theoretical point at which all molecular motion ceases. This is a fundamental concept in thermodynamics and has profound implications for various scientific fields, including physics, chemistry, and engineering.
Unlike Celsius and Fahrenheit, the Kelvin scale doesn't use negative values. This makes it exceptionally useful for calculations involving ideal gas laws and other thermodynamic relationships where negative temperatures would be nonsensical. Each degree Kelvin represents the same temperature change as a degree Celsius; the only difference lies in the zero point.
The relationship between Kelvin and Celsius is:
K = °C + 273.15
Standard Temperatures: Context Matters
As mentioned earlier, there isn't one single "standard temperature" in Kelvin universally applicable to all scenarios. The chosen standard depends heavily on the specific context:
1. Standard Ambient Temperature
For many everyday applications, particularly in chemistry and biology, 293.15 K (20°C, 68°F) often serves as a convenient reference point for "standard" or "room" temperature. It represents a comfortable and relatively stable temperature for many experiments and processes. This temperature is often considered as a baseline for numerous calculations, particularly in chemical kinetics where reaction rates are frequently temperature dependent.
2. Standard Temperature and Pressure (STP)
In chemistry and physics, Standard Temperature and Pressure (STP) is frequently used as a reference point for gas behavior. While the pressure component is well-defined (typically 1 atm or 101.325 kPa), the standard temperature has seen some variations historically.
-
Older STP: Often defined as 273.15 K (0°C). This definition is still encountered in older textbooks and literature.
-
Current IUPAC Recommendation: The International Union of Pure and Applied Chemistry (IUPAC) now recommends using 273.15 K (0°C) and 100 kPa as the standard temperature and pressure (STP). This newer definition aims for greater consistency and applicability across diverse conditions. This shift is noteworthy and highlights the evolving nature of scientific standards.
3. Standard Conditions for Gas Density
When calculating the density of gases, especially in industrial settings, standard temperature and pressure are crucial parameters. The choice of temperature within the standard conditions often depends on the specific industry and application but frequently centers around the 273.15 K (0°C) and 100 kPa benchmark established by IUPAC.
Why 273.15 K is Important
The temperature 273.15 K (0°C) holds special significance because it represents the freezing point of water at standard atmospheric pressure. This is a readily reproducible and easily understood reference point, making it a practical choice for defining standards. Furthermore, its use in calculating gas volumes and behavior under standard conditions provides a consistent and reliable baseline for scientific investigations. The readily accessible and reproducible nature of this temperature plays a significant role in its selection as a standard in a variety of scientific and engineering contexts.
Implications of Temperature on Various Processes
Understanding standard temperature, particularly in Kelvin, is critical across various scientific and engineering disciplines. Here are some key areas where its accurate determination is essential:
1. Chemical Reactions:
Reaction rates are highly temperature-sensitive. Many chemical reactions proceed much faster at higher temperatures due to increased kinetic energy of the reactant molecules. Knowing the standard temperature allows scientists to compare and predict reaction rates under controlled conditions. This is pivotal in designing chemical processes, optimising reaction yields, and ensuring safety.
2. Gas Laws:
The ideal gas law (PV = nRT) directly incorporates temperature (T) in Kelvin. Accurate temperature measurement is crucial for applying this fundamental law, which governs the behavior of gases under different conditions. This is particularly important in applications such as predicting the behavior of gases in engines or designing processes involving gas handling and storage.
3. Material Science:
Material properties, such as strength, conductivity, and phase transitions, are strongly influenced by temperature. Using standard temperature as a reference point is essential when characterizing and comparing materials' behavior. This understanding influences the selection of materials for specific applications, such as in aerospace or construction engineering.
4. Thermodynamics:
The Kelvin scale is fundamental in thermodynamics, providing a consistent basis for defining concepts such as entropy and enthalpy. Many thermodynamic calculations rely on absolute temperatures, making Kelvin essential for accurate predictions and analyses of energy transformations. This is critical in areas such as power generation, refrigeration, and chemical engineering.
5. Biology and Medicine:
While biological systems operate over a range of temperatures, standard temperatures are used in laboratory experiments to ensure reproducibility and comparability of results. Maintaining specific temperatures is crucial for cell culture, enzymatic reactions, and other biological processes. Medical equipment also often requires precise temperature control for calibration and accurate measurements.
Conclusion
The concept of "standard temperature in Kelvin" isn't a single, definitive value. Instead, it's context-dependent. While 273.15 K (0°C) frequently serves as a standard for STP and other established criteria, 293.15 K (20°C) is often used as a convenient reference for ambient conditions. Understanding the nuances of these values and their significance within different scientific and engineering disciplines is crucial for accurate calculations, reproducible experiments, and consistent results. The absolute nature of the Kelvin scale makes it indispensable for thermodynamic calculations and underscores its importance in understanding the fundamental relationships between temperature and matter. The ongoing refinements to standard definitions, such as the IUPAC recommendation for STP, highlight the dynamic nature of scientific progress and the constant striving for greater accuracy and consistency in measurements and standards.
Latest Posts
Latest Posts
-
Write The Equilibrium Constant Expression For This Reaction
Apr 01, 2025
-
What Is 3 6 In A Fraction
Apr 01, 2025
-
What Are Convection Currents And What Causes Them
Apr 01, 2025
-
Circumference Of A Circle With A Diameter Of 10
Apr 01, 2025
-
What Transition Metals Have A Fixed Charge
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is Standard Temperature In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
