What Happens To Atoms In A Chemical Reaction
listenit
Mar 21, 2025 · 6 min read
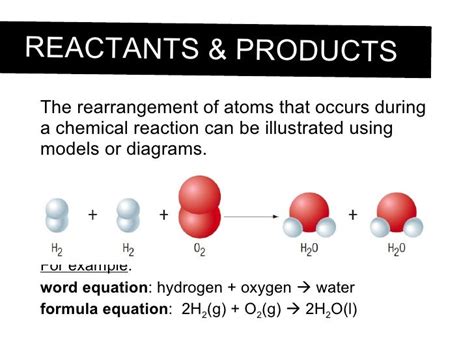
Table of Contents
What Happens to Atoms in a Chemical Reaction? A Deep Dive
Chemical reactions are the fundamental processes that govern the world around us, from the rusting of iron to the photosynthesis in plants. But what actually happens to the atoms involved in these reactions? Understanding this is key to understanding chemistry itself. This in-depth exploration will delve into the intricacies of atomic behavior during chemical reactions, covering concepts like electron rearrangement, bond breaking and formation, and the conservation of mass and atoms.
The Unchanging Atom: Conservation of Mass and Atoms
At the heart of every chemical reaction lies a fundamental principle: the law of conservation of mass. This law states that matter cannot be created or destroyed in a chemical reaction. While the arrangement of atoms changes dramatically, the total number of atoms of each element remains constant throughout the process. This is a cornerstone of chemical stoichiometry, allowing us to balance chemical equations and predict the amounts of reactants and products involved.
No Atoms Are Lost or Gained
Consider the simple reaction of hydrogen gas (H₂) and oxygen gas (O₂) to form water (H₂O):
2H₂ + O₂ → 2H₂O
In this reaction, two molecules of hydrogen react with one molecule of oxygen to produce two molecules of water. Let's count the atoms:
- Reactants: 4 hydrogen atoms (2 x 2) + 2 oxygen atoms (1 x 2) = 6 atoms total
- Products: 4 hydrogen atoms (2 x 2) + 2 oxygen atoms (2 x 1) = 6 atoms total
The number of hydrogen and oxygen atoms is the same on both sides of the equation. No atoms have been created or destroyed; they've simply rearranged themselves to form new molecules. This principle holds true for all chemical reactions, regardless of their complexity.
The Dance of Electrons: Rearrangement and Bond Formation
While atoms themselves remain intact during chemical reactions, their electrons are the primary players. It's the interaction and rearrangement of electrons that drive the breaking and formation of chemical bonds, ultimately transforming reactants into products.
Valence Electrons: The Key Players
The outermost electrons of an atom, known as valence electrons, are the ones most directly involved in chemical bonding. These electrons are loosely held and can be shared or transferred between atoms, leading to the formation of chemical bonds. The number of valence electrons determines an element's reactivity and the types of bonds it can form.
Ionic Bonds: Electron Transfer
In ionic bonds, one atom completely transfers one or more valence electrons to another atom. This transfer creates ions: positively charged cations (atoms that have lost electrons) and negatively charged anions (atoms that have gained electrons). The electrostatic attraction between these oppositely charged ions forms the ionic bond. A classic example is the formation of sodium chloride (NaCl) from sodium (Na) and chlorine (Cl). Sodium loses one electron to become Na⁺, while chlorine gains one electron to become Cl⁻. The resulting electrostatic attraction between Na⁺ and Cl⁻ forms the ionic bond in NaCl.
Covalent Bonds: Electron Sharing
In covalent bonds, atoms share valence electrons to achieve a more stable electron configuration. This sharing creates a stable bond between the atoms. The simplest example is the hydrogen molecule (H₂), where each hydrogen atom shares its single electron with the other, forming a stable covalent bond. Many organic molecules, such as methane (CH₄) and glucose (C₆H₁₂O₆), are held together by covalent bonds.
Metallic Bonds: A Sea of Electrons
In metallic bonds, valence electrons are delocalized and shared among a large number of atoms. This creates a "sea" of electrons that are free to move throughout the metal, resulting in the characteristic properties of metals such as electrical and thermal conductivity, malleability, and ductility.
Bond Breaking and Formation: The Energetics of Reactions
Chemical reactions involve the breaking of existing bonds in reactants and the formation of new bonds in products. This process is accompanied by changes in energy.
Activation Energy: The Energy Barrier
Before a reaction can occur, sufficient energy must be supplied to break the existing bonds in the reactants. This energy is called the activation energy. The activation energy represents an energy barrier that must be overcome for the reaction to proceed. This is why many reactions require heating or the presence of a catalyst. A catalyst lowers the activation energy, making the reaction occur faster.
Exothermic vs. Endothermic Reactions
Chemical reactions can be classified as either exothermic or endothermic based on their energy changes.
-
Exothermic reactions release energy to the surroundings. The energy released is usually in the form of heat, but it can also be in the form of light or sound. The products have lower energy than the reactants. Many combustion reactions are exothermic.
-
Endothermic reactions absorb energy from the surroundings. The products have higher energy than the reactants. Photosynthesis is an example of an endothermic reaction, as it requires energy from sunlight.
Beyond Simple Reactions: Complex Chemical Transformations
The principles discussed above apply to all chemical reactions, even the most complex ones. However, the intricacies of these reactions can be significantly more challenging to understand.
Reaction Mechanisms: Step-by-Step Breakdown
Many reactions don't occur in a single step. Instead, they proceed through a series of intermediate steps, called a reaction mechanism. Each step involves bond breaking and bond formation, and understanding the mechanism is crucial for predicting the outcome of the reaction and controlling its rate.
Catalysis: Accelerating Reactions
Catalysts are substances that increase the rate of a chemical reaction without being consumed themselves. They achieve this by providing an alternative reaction pathway with a lower activation energy. Enzymes, biological catalysts, are essential for countless reactions in living organisms.
Equilibrium: A Dynamic Balance
Many reactions are reversible, meaning that the products can react to reform the reactants. When the rates of the forward and reverse reactions are equal, the system is said to be in equilibrium. The equilibrium constant, K, quantifies the relative amounts of reactants and products at equilibrium.
Applications and Conclusion
Understanding what happens to atoms in a chemical reaction is fundamental to numerous fields, including:
- Medicine: Drug design and development rely heavily on understanding chemical reactions within the body.
- Materials Science: Creating new materials with specific properties involves carefully controlling chemical reactions.
- Environmental Science: Understanding chemical reactions is crucial for addressing environmental pollution and developing sustainable technologies.
- Food Science: Food processing involves numerous chemical reactions, impacting taste, texture, and preservation.
In conclusion, while atoms themselves remain unchanged during chemical reactions, their electrons are the driving force behind the transformation of reactants into products. The breaking and formation of chemical bonds, governed by the principles of conservation of mass and energy, dictate the outcome of these reactions. By understanding these fundamental concepts, we gain insight into the complexity and beauty of the chemical world surrounding us. The intricacies of electron rearrangement, bond energies, and reaction mechanisms provide a rich and ever-evolving field of study crucial to advancing knowledge in numerous scientific disciplines. Further exploration into specific reaction types, kinetics, and thermodynamics will provide even deeper insight into the fascinating world of chemical transformations.
Latest Posts
Latest Posts
-
Integral Of X Sqrt X 1
Mar 28, 2025
-
3 Main Ideas Of Cell Theory
Mar 28, 2025
-
What Are The Most Reactive Metals In The Periodic Table
Mar 28, 2025
-
Is Supports Combustion A Physical Or Chemical Property
Mar 28, 2025
-
What Is 6 Divided By 1 3
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Happens To Atoms In A Chemical Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
