What Functional Groups Are Present In All Amino Acids
listenit
Mar 26, 2025 · 7 min read
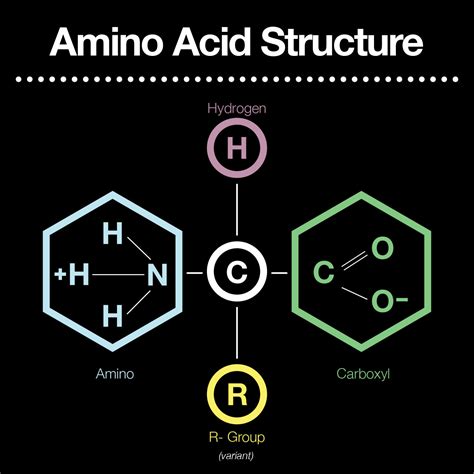
Table of Contents
What Functional Groups Are Present in All Amino Acids?
Amino acids, the building blocks of proteins, are organic molecules characterized by a specific set of functional groups. Understanding these groups is crucial to comprehending the diverse properties and functions of amino acids and the proteins they form. While the side chains (R groups) vary significantly among the 20 standard amino acids, giving them unique characteristics, all amino acids share three fundamental functional groups: an amino group (-NH2), a carboxyl group (-COOH), and a hydrogen atom (-H), all bonded to a central carbon atom called the alpha carbon. This consistent core structure underpins the fundamental chemistry of all amino acids. Let's delve deeper into each of these crucial functional groups and their contribution to the overall properties of amino acids.
The Amino Group (-NH2)
The amino group is a crucial functional group found in all amino acids. It consists of a nitrogen atom bonded to two hydrogen atoms. This group is basic, meaning it can accept a proton (H+) to form an ammonium ion (-NH3+). This property is central to the amino acid's ability to act as a weak base in aqueous solutions.
Properties and Significance of the Amino Group
-
Basicity: The lone pair of electrons on the nitrogen atom in the amino group readily accepts protons, leading to the formation of a positively charged ammonium ion. This ability to accept protons is fundamental to the amino acid's behavior in solutions of varying pH.
-
Hydrogen Bonding: The amino group's nitrogen atom can participate in hydrogen bonding, both as a hydrogen bond donor (providing a hydrogen atom) and a hydrogen bond acceptor (accepting a hydrogen bond from another molecule). This capability is vital for the secondary, tertiary, and quaternary structure of proteins, where hydrogen bonds help stabilize the intricate three-dimensional folding patterns.
-
Peptide Bond Formation: Perhaps the most crucial role of the amino group is in the formation of peptide bonds. During protein synthesis, the carboxyl group of one amino acid reacts with the amino group of another amino acid, releasing a water molecule and forming a peptide bond (amide linkage). This process links amino acids together to create polypeptide chains, the precursors to proteins.
-
Influence on Acid-Base Properties: The amino group's pKa value (the pH at which half of the amino groups are protonated) significantly impacts the overall charge and behavior of the amino acid at different pH levels. This is crucial for understanding how proteins function in various biological environments.
The Carboxyl Group (-COOH)
The carboxyl group, another fundamental component of all amino acids, consists of a carbon atom double-bonded to an oxygen atom and single-bonded to a hydroxyl group (-OH). This group is acidic, meaning it can donate a proton (H+), forming a carboxylate ion (-COO-). This contributes to the amino acid's ability to act as a weak acid in solution.
Properties and Significance of the Carboxyl Group
-
Acidity: The carboxyl group readily loses a proton in aqueous solution, forming a negatively charged carboxylate ion. This release of a proton is the reason amino acids can act as weak acids. The acidity is influenced by the presence of other functional groups within the amino acid.
-
Hydrogen Bonding: Like the amino group, the carboxyl group also participates in hydrogen bonding. The oxygen atoms in the carboxyl group can act as hydrogen bond acceptors, while the hydroxyl group can act as a hydrogen bond donor. These hydrogen bonds play a vital role in protein structure and stability.
-
Peptide Bond Formation: The carboxyl group is directly involved in the formation of peptide bonds. As mentioned earlier, the reaction between the carboxyl group of one amino acid and the amino group of another is a condensation reaction that releases water and creates the peptide bond linking amino acids together.
-
Influence on Acid-Base Properties: The pKa value of the carboxyl group is another key factor in determining the overall charge and behavior of the amino acid at different pH levels, significantly influencing protein function.
-
Metabolic Reactions: The carboxyl group is involved in various metabolic reactions, including the formation of acyl-CoA derivatives, essential intermediates in metabolic pathways.
The Hydrogen Atom (-H)
While seemingly simple, the hydrogen atom bonded to the alpha carbon plays a critical role in the overall structure and stereochemistry of amino acids. Although not a functional group in the same sense as the amino and carboxyl groups, its presence is essential.
Significance of the Hydrogen Atom
-
Chirality: The presence of four distinct substituents (amino group, carboxyl group, hydrogen atom, and R group) bonded to the alpha carbon makes most amino acids chiral molecules. This means they exist in two mirror-image forms, known as L- and D-isomers. Living organisms predominantly utilize L-amino acids in protein synthesis.
-
Structural Integrity: The hydrogen atom contributes to the overall three-dimensional structure of the amino acid, indirectly influencing its interactions with other molecules and its role in protein folding.
-
Metabolic Reactions: The hydrogen atom attached to the alpha carbon may participate in certain enzymatic reactions and metabolic processes.
The Alpha Carbon
The alpha carbon, the central carbon atom to which the amino group, carboxyl group, hydrogen atom, and R group are attached, is not a functional group itself. However, it is the structural backbone of every amino acid and its unique position ensures the unique chemical properties of each amino acid.
Importance of the Alpha Carbon
-
Chiral Center: For most amino acids, the alpha carbon acts as a chiral center. This chirality is critical for biological activity, as enzymes often exhibit stereospecificity, meaning they only interact with specific isomers.
-
Attachment Point: The alpha carbon serves as the attachment point for all the other functional groups defining the amino acid. Its tetrahedral geometry influences the spatial arrangement of these groups and their interactions.
-
Backbone of Amino Acid: The alpha carbon along with its attached groups forms the backbone of the amino acid, dictating the direction and arrangement of polypeptide chains during protein synthesis.
The R Group (Side Chain) – The Unique Identifier
The R group, or side chain, is the fourth substituent bonded to the alpha carbon. It is this R group that provides each of the 20 standard amino acids its unique chemical properties. R groups vary greatly in size, shape, charge, and polarity, significantly affecting the overall properties and function of the amino acids and the proteins they comprise.
The Diversity of R Groups
The diversity in R groups determines whether an amino acid is:
-
Nonpolar (hydrophobic): These amino acids have R groups that are primarily composed of hydrocarbons, making them insoluble in water. Examples include glycine, alanine, valine, leucine, isoleucine, methionine, phenylalanine, tryptophan, and proline.
-
Polar (hydrophilic): These amino acids have R groups with polar functional groups like hydroxyl (-OH), sulfhydryl (-SH), or amide groups. They readily interact with water. Examples include serine, threonine, cysteine, asparagine, glutamine, and tyrosine.
-
Charged (acidic or basic): These amino acids have R groups that carry a net charge at physiological pH. Acidic amino acids have negatively charged carboxyl groups in their side chains (aspartic acid and glutamic acid). Basic amino acids have positively charged amino groups in their side chains (lysine, arginine, and histidine).
The variation in R group properties directly impacts protein structure and function. Hydrophobic R groups tend to cluster together in the interior of proteins, whereas hydrophilic R groups are often found on the protein surface interacting with the aqueous environment. Charged R groups can participate in ionic interactions, further stabilizing protein structure or mediating protein interactions.
Conclusion: The Foundation of Protein Structure and Function
The consistent presence of the amino group, carboxyl group, and hydrogen atom on the alpha carbon forms the fundamental structural scaffold of all amino acids. This core structure, coupled with the diversity of the R group, allows for the vast array of amino acid properties and, ultimately, the remarkable diversity of protein structure and function. Understanding these functional groups and their interplay is fundamental to comprehending the complex world of proteins and their crucial role in living organisms. From the simple formation of peptide bonds to the intricate folding patterns that determine protein activity, the functional groups of amino acids provide the basis for life itself. The intricate balance between these groups and the unique contribution of the R groups allows for the immense diversity and specificity of biological processes.
Latest Posts
Latest Posts
-
Whats The Square Root Of 23
Mar 29, 2025
-
What Is The Decimal For 6 10
Mar 29, 2025
-
What Is The Square Root Of 2000
Mar 29, 2025
-
What Are 4 Agents Of Erosion
Mar 29, 2025
-
Molar Mass Of Sr No3 2
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Functional Groups Are Present In All Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
