What Element Is Found In Carbohydrates
listenit
Apr 01, 2025 · 6 min read
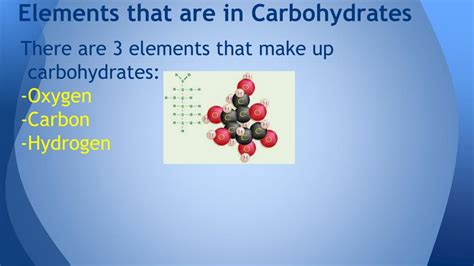
Table of Contents
What Element is Found in Carbohydrates? A Deep Dive into Carbohydrate Composition
Carbohydrates, often the first energy source our bodies tap into, are essential for life. But what exactly are they? At their core, carbohydrates are organic compounds composed primarily of just three elements: carbon (C), hydrogen (H), and oxygen (O). While seemingly simple, the arrangement of these atoms creates the incredible diversity of carbohydrates found in nature, from the simple sugars in fruits to the complex starches in potatoes and the structural fibers in plants. This article will delve deep into the composition of carbohydrates, exploring the roles of each element, the different types of carbohydrates, and the implications of their chemical structure for their biological function.
The Trio: Carbon, Hydrogen, and Oxygen
The basic building block of all carbohydrates is a simple sugar unit called a monosaccharide. These monosaccharides are characterized by their specific ratio of carbon, hydrogen, and oxygen atoms. The general formula for a monosaccharide is (CH₂O)ₙ, where 'n' represents the number of carbon atoms. This formula beautifully illustrates the fundamental composition: equal parts carbon and oxygen, and twice as many hydrogen atoms.
Let's break down the roles of each element:
Carbon (C): The Backbone
Carbon forms the structural backbone of all carbohydrates. Its unique ability to form four strong covalent bonds allows it to create long chains, branched chains, and ring structures. These structures provide the framework for the diverse shapes and sizes of carbohydrate molecules. The carbon atoms in monosaccharides are numbered to allow for precise descriptions of the molecule's structure and the position of other atoms like hydroxyl (-OH) groups.
Hydrogen (H): Influencing Reactivity and Structure
Hydrogen atoms are attached to every carbon atom in the carbohydrate molecule, except for one which is double bonded to an oxygen atom to form a carbonyl group (C=O). The presence of hydrogen atoms significantly impacts the reactivity of carbohydrates. The hydroxyl (-OH) groups, formed by the bond between a hydrogen and an oxygen atom, are crucial for many biochemical reactions involving carbohydrates. These hydroxyl groups are involved in hydrogen bonding, which plays a critical role in determining the three-dimensional structure of complex carbohydrates.
Oxygen (O): Key to Function and Solubility
Oxygen atoms are present in carbohydrates primarily as part of hydroxyl (-OH) groups and the carbonyl group (C=O). The carbonyl group can be either an aldehyde (at the end of the carbon chain) or a ketone (within the carbon chain), which distinguishes between aldoses and ketoses, two important classes of monosaccharides. Oxygen’s electronegativity influences the polarity of the carbohydrate molecule, making many carbohydrates soluble in water. This solubility is essential for their transport and use within the body.
The Diverse World of Carbohydrates
The seemingly simple formula (CH₂O)ₙ belies the astounding diversity of carbohydrates found in nature. This diversity arises from variations in:
- Chain length: Monosaccharides are the simplest, with 3 to 7 carbon atoms. Disaccharides are formed by linking two monosaccharides, and polysaccharides contain long chains of monosaccharides.
- Branching: Carbohydrate chains can be linear or branched, creating different three-dimensional structures with differing properties.
- Ring structures: In aqueous solutions, monosaccharides usually form ring structures, further adding complexity to their shapes.
- Types of monosaccharides: Different monosaccharides (glucose, fructose, galactose, etc.) have distinct arrangements of their carbon, hydrogen, and oxygen atoms, impacting their properties and functions.
- Glycosidic bonds: The bonds connecting monosaccharides together in disaccharides and polysaccharides are crucial, influencing their digestibility and properties.
Types of Carbohydrates based on Complexity:
-
Monosaccharides: These are the simplest carbohydrates, including glucose (blood sugar), fructose (fruit sugar), and galactose (found in milk). They are the building blocks of all other carbohydrates.
-
Disaccharides: Formed by the joining of two monosaccharides through a glycosidic bond, examples include sucrose (table sugar, glucose + fructose), lactose (milk sugar, glucose + galactose), and maltose (malt sugar, glucose + glucose).
-
Oligosaccharides: Contain a small number (3-10) of monosaccharides linked together. They are found in various foods and play a role in digestion and gut health.
-
Polysaccharides: These are large polymers composed of long chains of monosaccharides. Important polysaccharides include:
- Starch: A storage polysaccharide in plants, composed mainly of amylose and amylopectin, both made up of glucose units. Starch serves as a primary source of energy for humans.
- Glycogen: The storage polysaccharide in animals, similar to starch but with more branching. It is stored primarily in the liver and muscles.
- Cellulose: A structural polysaccharide in plants, forming the cell walls. Humans cannot digest cellulose, but it provides dietary fiber, crucial for digestive health.
- Chitin: A structural polysaccharide found in the exoskeletons of insects and crustaceans, and in fungal cell walls.
The Importance of Carbohydrate Structure
The structure of a carbohydrate molecule directly dictates its function. For instance:
- Linear vs. Branched: Branched polysaccharides like glycogen can be quickly broken down to release glucose, making them ideal for energy storage. Linear polysaccharides like cellulose are more resistant to breakdown, providing structural support.
- Types of Glycosidic Bonds: The type of glycosidic bond (alpha or beta) determines whether a polysaccharide is digestible by humans. Alpha-glycosidic bonds, found in starch and glycogen, are easily broken down by human enzymes. Beta-glycosidic bonds, present in cellulose, are not digestible by humans.
- Ring Configuration: The specific ring configuration of a monosaccharide (alpha or beta anomers) influences its interactions with other molecules and its ability to form glycosidic bonds.
Carbohydrates and Human Health
Carbohydrates are an essential macronutrient, providing the body with energy through glucose metabolism. Different types of carbohydrates have varying effects on health:
- Simple carbohydrates: These are rapidly digested and absorbed, leading to quick spikes in blood sugar. Excessive consumption can contribute to weight gain and other health problems.
- Complex carbohydrates: These are digested more slowly, providing a sustained release of energy and helping to regulate blood sugar levels. They are often rich in fiber, which promotes digestive health.
- Dietary fiber: Non-digestible carbohydrates that contribute to gut health, help regulate bowel movements, and can aid in weight management.
Conclusion
The seemingly simple composition of carbohydrates—carbon, hydrogen, and oxygen—underpins a vast array of molecules with diverse structures and functions. Understanding the arrangement of these three elements within monosaccharides, disaccharides, and polysaccharides is fundamental to comprehending the roles carbohydrates play in biological systems, from providing energy to providing structural support. By appreciating the relationship between carbohydrate structure and function, we gain valuable insights into their importance in human health and nutrition. Further research continues to unravel the complexities of carbohydrate chemistry and their far-reaching impacts on living organisms. This knowledge is essential for developing innovative strategies in agriculture, medicine, and biomaterials science, highlighting the enduring significance of these ubiquitous molecules.
Latest Posts
Latest Posts
-
12x 4y 20 Solve For Y
Apr 02, 2025
-
Most Reactive Group On The Periodic Table
Apr 02, 2025
-
How To Solve Multi Step Inequalities
Apr 02, 2025
-
Percent Composition Of Mg No3 2
Apr 02, 2025
-
How To Convert 3 8 Into A Decimal
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Element Is Found In Carbohydrates . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
