What Do The Coefficients In A Chemical Equation Represent
listenit
Mar 30, 2025 · 6 min read
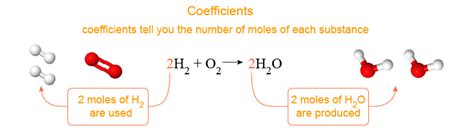
Table of Contents
What Do the Coefficients in a Chemical Equation Represent?
Chemical equations are the shorthand language of chemistry, providing a concise way to describe chemical reactions. Understanding these equations is crucial for predicting reaction outcomes, calculating yields, and generally comprehending the stoichiometry of chemical processes. A key component of chemical equations is the coefficients—the numbers placed before chemical formulas—and understanding their meaning is fundamental to mastering chemical calculations. This article delves deep into the significance of coefficients in chemical equations, exploring their role in representing the relative amounts of reactants and products, and their connection to the law of conservation of mass.
The Fundamental Role of Coefficients: Representing Moles and Ratios
Coefficients in a balanced chemical equation represent the relative number of moles of each substance involved in the reaction. They don't represent individual atoms or molecules directly, but rather the relative quantities of reactants consumed and products formed. This is a crucial distinction. For example, consider the combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
In this equation:
- 1 represents one mole of methane (CH₄).
- 2 represents two moles of oxygen gas (O₂).
- 1 represents one mole of carbon dioxide (CO₂).
- 2 represents two moles of water (H₂O).
The coefficients tell us that one mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide and two moles of water. The ratio of reactants to products is precisely defined by these coefficients. This ratio is absolutely critical for performing stoichiometric calculations, which are used to determine the amounts of reactants needed or the amounts of products formed in a chemical reaction.
Beyond Moles: Understanding the Ratio
While coefficients directly represent moles, their deeper significance lies in the ratio they establish between reactants and products. This ratio is constant for a given chemical reaction under specific conditions (temperature, pressure etc.). This ratio allows us to scale up or down the reaction without changing the fundamental relationship between the reactants and products.
For instance, in the methane combustion example:
- We could react 2 moles of CH₄ with 4 moles of O₂ to produce 2 moles of CO₂ and 4 moles of H₂O.
- Or we could react 0.5 moles of CH₄ with 1 mole of O₂ to produce 0.5 moles of CO₂ and 1 mole of H₂O.
Notice that the ratio of CH₄:O₂:CO₂:H₂O remains 1:2:1:2 regardless of the scale. This is a direct consequence of the coefficients and their representation of the molar ratios within the reaction.
Coefficients and the Law of Conservation of Mass
The fundamental principle underpinning balanced chemical equations is the law of conservation of mass. This law states that matter cannot be created or destroyed in a chemical reaction; it only changes form. The coefficients in a balanced chemical equation ensure that the law of conservation of mass is obeyed.
Let's analyze the methane combustion equation again:
CH₄ + 2O₂ → CO₂ + 2H₂O
By examining the number of atoms of each element on both sides of the equation, we can verify mass conservation:
- Carbon (C): 1 carbon atom on the left (in CH₄) and 1 carbon atom on the right (in CO₂).
- Hydrogen (H): 4 hydrogen atoms on the left (in CH₄) and 4 hydrogen atoms on the right (in 2H₂O).
- Oxygen (O): 4 oxygen atoms on the left (in 2O₂) and 4 oxygen atoms on the right (in CO₂ and 2H₂O).
Because the number of atoms of each element is the same on both sides of the equation, the total mass remains constant throughout the reaction. This is a direct consequence of the carefully chosen coefficients, which balance the equation. An unbalanced equation would violate the law of conservation of mass, implying the creation or destruction of matter, which is impossible.
Balancing Chemical Equations and the Significance of Coefficients
Balancing chemical equations is the process of adjusting coefficients to ensure that the number of atoms of each element is equal on both the reactant and product sides. This process is crucial because it allows us to accurately represent the stoichiometry of a reaction.
Balancing equations involves a systematic approach, often involving trial and error. However, the underlying principle remains consistent: ensuring that the coefficients maintain the conservation of mass and correctly reflect the molar ratios.
Let's consider a slightly more complex example, the reaction between iron and oxygen to form iron(III) oxide:
Fe + O₂ → Fe₂O₃
This equation is unbalanced. To balance it, we must adjust the coefficients:
4Fe + 3O₂ → 2Fe₂O₃
Now, let's check the atom balance:
- Iron (Fe): 4 iron atoms on the left and 4 iron atoms on the right (in 2Fe₂O₃).
- Oxygen (O): 6 oxygen atoms on the left (in 3O₂) and 6 oxygen atoms on the right (in 2Fe₂O₃).
The equation is now balanced, reflecting the correct molar ratios and adhering to the law of conservation of mass.
Applications of Coefficients in Chemical Calculations
The coefficients in balanced chemical equations are essential for performing various stoichiometric calculations. These calculations allow us to determine:
- Theoretical Yield: The maximum amount of product that can be formed from a given amount of reactant, based on the stoichiometric ratios defined by the coefficients.
- Limiting Reactant: The reactant that is completely consumed first in a reaction, thus limiting the amount of product that can be formed.
- Percent Yield: The actual yield of a reaction (the amount of product actually obtained) expressed as a percentage of the theoretical yield.
- Reactant Needed: The quantity of reactants required to produce a specified amount of product.
These calculations are routinely used in various chemical applications, from industrial chemical processes to laboratory experiments. The accuracy of these calculations depends entirely on the correct balancing of the chemical equation and the correct interpretation of the coefficients.
Beyond Simple Reactions: Complex Scenarios and Coefficients
While the examples above involve relatively simple reactions, the principles remain the same for more complex chemical equations involving multiple reactants and products. The coefficients always represent the relative molar ratios, ensuring mass conservation and providing the basis for accurate stoichiometric calculations.
For instance, consider the reaction for the synthesis of ammonia:
N₂ + 3H₂ → 2NH₃
The coefficients indicate that one mole of nitrogen gas reacts with three moles of hydrogen gas to produce two moles of ammonia. This ratio is fundamental in determining the yield of ammonia in an industrial setting.
Similarly, in reactions involving ionic compounds, the coefficients represent the relative number of moles of ionic species reacting and forming. The balanced equation must ensure the conservation of charge as well as mass.
Conclusion: Coefficients – The Key to Understanding Chemical Reactions
In summary, the coefficients in a balanced chemical equation represent the relative number of moles of each substance involved in a reaction. Their primary significance lies in their ability to define the molar ratios between reactants and products. These ratios are essential for ensuring adherence to the law of conservation of mass and are fundamental for performing accurate stoichiometric calculations. Understanding the meaning and application of coefficients is, therefore, crucial for anyone studying or working with chemical reactions, whether in an academic or industrial context. The ability to interpret and utilize the information encoded within the coefficients is a cornerstone of chemical understanding and problem-solving.
Latest Posts
Latest Posts
-
What Is The Decimal For 11 20
Apr 01, 2025
-
Aliyah Had 24 To Spend On Seven Pencils
Apr 01, 2025
-
What Is The Length Of Ef
Apr 01, 2025
-
What Is The Square Root Of 6 25
Apr 01, 2025
-
Consider The Face Centered Cubic Unit Cell Shown In This Image
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Do The Coefficients In A Chemical Equation Represent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
