Consider The Face-centered Cubic Unit Cell Shown In This Image
listenit
Apr 01, 2025 · 6 min read
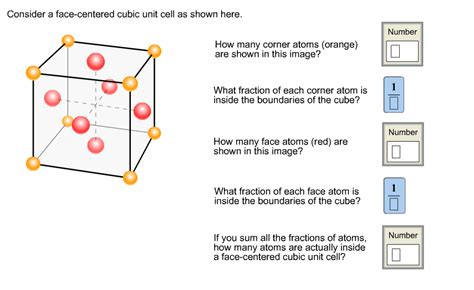
Table of Contents
Decoding the Face-Centered Cubic (FCC) Unit Cell: A Deep Dive
The face-centered cubic (FCC) unit cell, a fundamental building block in crystallography, represents a highly efficient and common arrangement of atoms in numerous metallic and some ionic structures. Understanding its structure, properties, and implications is crucial for materials science, chemistry, and various engineering disciplines. This article will delve deep into the FCC unit cell, exploring its characteristics, calculating its properties, and highlighting its significance in material behavior.
Understanding the FCC Structure: Atoms and Geometry
The FCC unit cell, as the name suggests, is a cube with atoms situated at each of its eight corners and at the center of each of its six faces. This seemingly simple arrangement gives rise to a surprisingly complex and robust structure with unique properties.
Key features of the FCC structure:
- Cubic symmetry: The unit cell possesses cubic symmetry, meaning it has equal edge lengths (a) and 90° angles between its axes. This high degree of symmetry impacts its physical properties, making it isotropic in many respects.
- Coordination number: Each atom in an FCC lattice is surrounded by twelve nearest neighbors – six in its own plane and three in each plane above and below. This high coordination number contributes to the strong bonding and relatively high density observed in FCC materials.
- Atomic Packing Factor (APF): The APF represents the fraction of the unit cell volume occupied by atoms. For an FCC structure, the APF is exceptionally high, approximately 0.74. This signifies that atoms are packed very efficiently within the FCC lattice, leading to high density materials. We will explore the calculation of APF later in this article.
- Basis: The FCC lattice can be described as a simple cubic lattice with a basis of four atoms – one at each corner and one at the center of the cube face.
Calculating Key Properties of the FCC Unit Cell
Several critical properties can be derived from the FCC unit cell's geometry. Let's examine some key calculations:
1. Number of Atoms per Unit Cell:
The atoms at the corners are shared among eight adjacent unit cells. Therefore, each corner atom contributes 1/8 of an atom to the unit cell. With eight corners, this contributes 8 * (1/8) = 1 atom. The six face-centered atoms each contribute 1/2 an atom to the unit cell, resulting in 6 * (1/2) = 3 atoms. In total, the FCC unit cell contains 1 + 3 = 4 atoms.
2. Atomic Packing Factor (APF):
The APF is calculated as the ratio of the volume occupied by atoms to the total volume of the unit cell.
- Volume occupied by atoms: Assuming atoms are hard spheres with radius 'r', the volume of a single atom is (4/3)πr³. Since there are four atoms per unit cell, the total volume occupied is 4 * (4/3)πr³.
- Total volume of the unit cell: The volume of the cube is a³. The relationship between the edge length 'a' and the atomic radius 'r' in an FCC structure is a = 2√2r. Therefore, the total volume is (2√2r)³.
Therefore, the APF is:
APF = [4 * (4/3)πr³] / [(2√2r)³] = π√2 / 6 ≈ 0.74
This high APF contributes to the density of FCC metals.
3. Density Calculation:
The density (ρ) of a material with an FCC structure can be calculated using the following formula:
ρ = (n * A) / (V * N<sub>A</sub>)
Where:
- n = number of atoms per unit cell (4 for FCC)
- A = atomic weight of the element (g/mol)
- V = volume of the unit cell (a³)
- N<sub>A</sub> = Avogadro's number (6.022 x 10²³ atoms/mol)
This formula allows us to calculate the theoretical density of an FCC material if we know the atomic weight and atomic radius.
Common FCC Metals and Their Properties
Many common metals crystallize in the FCC structure. This structure’s efficiency in packing atoms leads to properties advantageous for various applications.
- Aluminum (Al): Lightweight, corrosion-resistant, and highly ductile, aluminum finds extensive use in aerospace, packaging, and construction.
- Copper (Cu): Excellent electrical and thermal conductivity makes copper a vital material in electrical wiring, plumbing, and heat exchangers.
- Gold (Au): Known for its inertness, malleability, and ductility, gold is used extensively in jewelry, electronics, and dentistry.
- Silver (Ag): Similar to copper, silver exhibits excellent electrical conductivity, making it valuable in electronics and specialized applications.
- Nickel (Ni): Nickel's strength, corrosion resistance, and magnetic properties make it important in various alloys, catalysts, and coatings.
- Platinum (Pt): Platinum is a highly resistant noble metal used in jewelry, catalytic converters, and high-temperature applications.
- Lead (Pb): While not always desirable, lead’s softness and malleability, previously used in lead-acid batteries and plumbing.
Implications of FCC Structure on Material Properties
The FCC structure significantly influences various material properties:
- Ductility and Malleability: The close-packed arrangement allows for relatively easy slip along certain crystallographic planes, resulting in high ductility and malleability. This means FCC metals can be easily deformed without fracturing.
- High Density: The high APF leads to a high density, which is beneficial in some applications but can be a disadvantage in others where lightweight materials are required.
- Electrical and Thermal Conductivity: The delocalized electrons in metallic FCC structures contribute to their excellent electrical and thermal conductivity.
- Strength: While not the strongest crystal structure, FCC metals can be strengthened through alloying, cold working, and other metallurgical processes.
- Stacking Faults: The FCC structure can exhibit stacking faults, where the stacking sequence of close-packed planes is interrupted, impacting its mechanical properties.
Advanced Concepts and Applications
Beyond the basics, several advanced concepts are related to FCC structures:
- Alloying: The addition of other elements can significantly alter the properties of FCC metals. Alloying can increase strength, corrosion resistance, or other desirable properties.
- Grain Boundaries: FCC materials are polycrystalline, meaning they consist of numerous grains with different orientations. Grain boundaries affect the mechanical properties and can be manipulated through various processing techniques.
- Phase Transformations: FCC metals can undergo phase transformations under certain conditions, leading to changes in their crystal structure and properties.
- Nanomaterials: The study of FCC nanomaterials explores the unique properties of FCC structures at the nanoscale, with potential applications in electronics, catalysis, and medicine.
Conclusion: The Significance of the FCC Unit Cell
The face-centered cubic unit cell is a foundational concept in materials science. Its efficient packing arrangement leads to several unique properties that make FCC metals vital in a wide range of applications. Understanding the FCC structure, its properties, and its influence on material behavior is essential for developing new materials and optimizing existing ones for diverse engineering and technological needs. This detailed exploration of the FCC unit cell provides a robust foundation for further study in the realm of materials science and crystallography. Further research can delve into specific FCC metal properties and their influence on material selection and applications.
Latest Posts
Latest Posts
-
How To Convert 3 8 Into A Decimal
Apr 02, 2025
-
Natural Resources Of The Northeast Region
Apr 02, 2025
-
How Many Unpaired Electrons Are In Sulfur
Apr 02, 2025
-
Whats The Square Root Of 69
Apr 02, 2025
-
What Is The Least Common Multiple Of 10 And 15
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Consider The Face-centered Cubic Unit Cell Shown In This Image . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
