What Are The Components Of Atp
listenit
Mar 15, 2025 · 6 min read
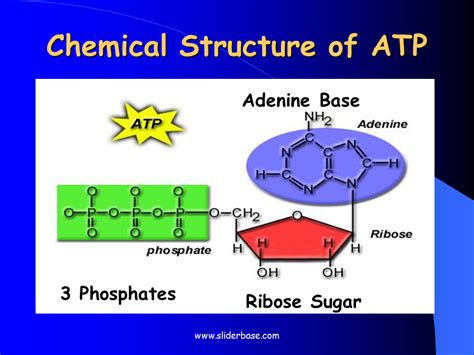
Table of Contents
What are the Components of ATP? Unraveling the Energy Currency of Life
Adenosine triphosphate (ATP) is the fundamental energy currency of all living cells. Its crucial role in powering countless cellular processes makes understanding its components essential for grasping the intricacies of biology. This comprehensive article delves deep into the structure of ATP, explaining its individual components and how they contribute to its remarkable energy-carrying capacity. We will also explore the synthesis and hydrolysis of ATP, highlighting its dynamic role in cellular metabolism.
The Building Blocks of ATP: Adenine, Ribose, and Phosphate Groups
ATP is a relatively small molecule, yet its impact on life is monumental. It's composed of three main components:
-
Adenine: A purine base, adenine is a nitrogenous heterocyclic aromatic organic compound. It's a crucial component of DNA and RNA, forming base pairs with thymine (in DNA) and uracil (in RNA). In ATP, adenine's role is primarily to provide a specific binding site for enzymes involved in ATP metabolism. Its planar structure contributes to the overall stability of the molecule.
-
Ribose: A five-carbon sugar (pentose), ribose forms the backbone of the ATP molecule. Specifically, it's a β-D-ribose, meaning the hydroxyl group on the anomeric carbon is in the beta configuration. This specific configuration is critical for the proper spatial arrangement of the phosphate groups and adenine, influencing the molecule's reactivity and interactions with enzymes. The ribose ring provides a structural framework that connects adenine to the phosphate groups.
-
Phosphate Groups: This is where the energy-carrying magic happens. ATP contains three phosphate groups, linked together by high-energy phosphoanhydride bonds. These bonds are often described as "high-energy" because a significant amount of free energy is released when they are hydrolyzed (broken). This energy release fuels numerous cellular processes. The three phosphate groups are sequentially labeled as alpha (α), beta (β), and gamma (γ), starting from the ribose. The bonds between these phosphate groups are the key to ATP's energy storage and release.
The Significance of High-Energy Phosphoanhydride Bonds
The phosphoanhydride bonds linking the phosphate groups are the cornerstone of ATP's function. Several factors contribute to their high energy content:
-
Electrostatic Repulsion: The negatively charged phosphate groups strongly repel each other. This repulsion creates an unstable state, storing potential energy in the bonds. When the bond is broken, this repulsion is relieved, releasing energy.
-
Resonance Stabilization: The products of ATP hydrolysis (ADP and inorganic phosphate) are more resonance-stabilized than ATP itself. Resonance stabilization refers to the delocalization of electrons, increasing the stability of the molecule. The increased stability of the products contributes to the energy released during hydrolysis.
-
Solvation: The products of hydrolysis are better solvated (surrounded by water molecules) than ATP. This increased solvation also contributes to the overall energy released during the reaction.
These factors collectively make the phosphoanhydride bonds in ATP exceptionally high-energy, making them ideal for driving numerous cellular processes that require energy input.
ATP Hydrolysis: Releasing Energy for Cellular Work
The energy stored within ATP's phosphate bonds is released through a process called hydrolysis. Hydrolysis involves the breaking of a chemical bond using a water molecule. In the context of ATP, this typically involves the removal of the terminal (γ) phosphate group:
ATP + H₂O → ADP + Pi + Energy
Where:
- ATP is adenosine triphosphate
- ADP is adenosine diphosphate
- Pi is inorganic phosphate
This reaction releases a considerable amount of free energy, approximately -7.3 kcal/mol under standard conditions. This energy is not directly used to power cellular processes; instead, it's coupled to other reactions, making them thermodynamically favorable. This coupling is facilitated by enzymes that use the energy released from ATP hydrolysis to drive endergonic (energy-requiring) reactions.
Examples of ATP-Driven Cellular Processes
ATP hydrolysis powers a vast array of cellular activities, including:
-
Muscle Contraction: The sliding filament mechanism responsible for muscle contraction relies heavily on ATP hydrolysis to provide the energy needed for myosin to interact with actin filaments.
-
Active Transport: The movement of molecules against their concentration gradients, a process crucial for maintaining cellular homeostasis, often requires ATP hydrolysis to power membrane pumps (e.g., sodium-potassium pump).
-
Biosynthesis: The synthesis of complex molecules, like proteins and nucleic acids, from simpler building blocks requires energy, supplied by ATP hydrolysis.
-
Signal Transduction: Cellular signaling pathways often involve ATP-dependent phosphorylation of proteins, altering their activity and mediating cellular responses.
-
Nerve Impulse Transmission: The propagation of nerve impulses depends on ATP-driven ion transport across neuronal membranes.
-
Cell Division: The intricate process of cell division, including DNA replication and chromosome segregation, demands significant energy from ATP hydrolysis.
These are only a few examples, and the versatility of ATP as an energy source underscores its importance in sustaining life.
ATP Synthesis: Regenerating the Energy Currency
Constantly using ATP would quickly deplete the cell's energy stores. Therefore, cells have evolved highly efficient mechanisms for regenerating ATP. The primary pathways for ATP synthesis are:
-
Oxidative Phosphorylation: This process takes place in the mitochondria and is the most significant source of ATP in aerobic organisms. It involves the electron transport chain and chemiosmosis, utilizing the energy released from the oxidation of glucose and other fuels to generate a proton gradient across the mitochondrial membrane. This gradient is then used to drive ATP synthesis by ATP synthase, an enzyme that acts as a molecular turbine.
-
Substrate-Level Phosphorylation: This less efficient method involves the direct transfer of a phosphate group from a substrate molecule to ADP, forming ATP. It occurs during glycolysis and the citric acid cycle, yielding a smaller amount of ATP compared to oxidative phosphorylation.
-
Photophosphorylation: This pathway, unique to photosynthetic organisms, utilizes light energy to drive ATP synthesis. Light energy is captured by chlorophyll and used to create a proton gradient across the thylakoid membrane in chloroplasts, similar to oxidative phosphorylation. This gradient then drives ATP synthesis via ATP synthase.
ATP and Cellular Regulation
The concentration of ATP and its related molecules (ADP and AMP) acts as a crucial signal for cellular regulation. The ATP/ADP ratio reflects the cell's energy status. High ATP levels generally indicate sufficient energy, while low ATP levels and elevated ADP/AMP levels signal energy depletion. These changes in energy status trigger various regulatory mechanisms, adjusting metabolic pathways to meet the cell's energy demands. For example, high ATP levels can inhibit certain enzymes involved in energy production, while low ATP levels stimulate these enzymes.
Conclusion: ATP – The Master Molecule of Life
Adenosine triphosphate, with its simple yet elegant structure, plays a central and irreplaceable role in all living organisms. Understanding its components – adenine, ribose, and the three phosphate groups, with their high-energy bonds – is fundamental to understanding how cells function. The dynamic interplay between ATP hydrolysis and synthesis ensures a constant supply of energy to power the countless biochemical reactions that maintain life. The importance of ATP extends beyond merely providing energy; its concentration also serves as a vital signal regulating cellular metabolism, demonstrating its central role in cellular homeostasis and survival. Further exploration of ATP's functions remains a rich area of biological research, continuing to reveal the complexities of this remarkable molecule.
Latest Posts
Latest Posts
-
What Is The Difference Between Ion And Atom
Mar 15, 2025
-
What Is 2 3 Of 10
Mar 15, 2025
-
1 1 3 Cups Divided By 2
Mar 15, 2025
-
How To Find The Ph At The Equivalence Point
Mar 15, 2025
-
How Long Would It Take Light To Reach Saturn
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Are The Components Of Atp . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
