What Are Horizontal Rows On The Periodic Table Called
listenit
Mar 24, 2025 · 6 min read
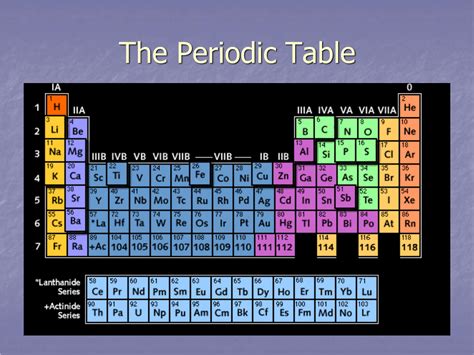
Table of Contents
What Are Horizontal Rows on the Periodic Table Called? A Deep Dive into Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. While many are familiar with its layout, a fundamental question often arises: what are the horizontal rows on the periodic table called? The answer is simple: periods. But understanding periods goes far beyond a simple label; they reveal profound insights into the behavior and properties of elements. This article delves deep into the concept of periods, exploring their structure, significance, and the underlying principles that govern their properties.
Understanding Periods: A Structural Overview
The periodic table arranges elements in a grid-like structure. Vertically, we have groups or families, elements sharing similar chemical properties. Horizontally, we encounter periods, which represent elements with the same number of electron shells. This seemingly simple arrangement holds immense significance.
Each period corresponds to a principal quantum number (n) in the electron configuration of the atoms. The first period (n=1) has only two elements, hydrogen and helium, as only the 1s orbital can be filled. As we move down the table to higher periods, the number of elements increases significantly. This is due to the increasing number of orbitals available at higher energy levels. For example:
- Period 1: Contains only two elements (Hydrogen and Helium) filling the 1s orbital.
- Period 2: Eight elements filling the 2s and 2p orbitals.
- Period 3: Eight elements filling the 3s and 3p orbitals.
- Period 4: Eighteen elements, including the filling of the 3d orbitals.
- Period 5: Eighteen elements, similarly including the filling of the 4d orbitals.
- Period 6: Thirty-two elements, including the filling of the 4f and 5d orbitals.
- Period 7: Currently incomplete, but expected to contain 32 elements, including the filling of the 5f and 6d orbitals.
This increase in element number reflects the expansion of the electron cloud and the availability of more complex electron configurations.
The Significance of Electron Shells and Periodicity
The number of electron shells directly correlates to the period an element resides in. Elements within the same period share the same number of electron shells, but their outermost electron configurations differ, leading to varying chemical properties. This is the essence of periodicity: the repeating pattern of properties observed across periods.
Atomic Radius Trends within Periods
As we move across a period from left to right, the atomic radius generally decreases. This is primarily because the number of protons in the nucleus increases, enhancing the positive charge and attracting the electrons more strongly. Consequently, the electron cloud is pulled closer to the nucleus, resulting in a smaller atomic radius. This trend is consistent across all periods, albeit with slight variations depending on electron configuration specifics.
Ionization Energy Trends within Periods
Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. The increased nuclear charge mentioned above makes it harder to remove an electron, requiring more energy. Exceptions to this trend exist, especially when the added electron enters a new subshell (e.g., from p to d orbitals).
Electronegativity Trends within Periods
Electronegativity, the ability of an atom to attract electrons in a chemical bond, also generally increases across a period. The increased nuclear charge leads to a stronger pull on shared electrons within a chemical bond. This trend is crucial in understanding the types of bonds (ionic, covalent, polar covalent) that form between elements.
Metallic Character Trends within Periods
The metallic character of elements generally decreases across a period. Elements on the left side of a period tend to be more metallic, readily losing electrons to form positive ions. Elements on the right side are less metallic, often gaining electrons to form negative ions or sharing electrons in covalent bonds. This trend is reflected in the properties of elements like conductivity, malleability, and ductility.
The Lanthanides and Actinides: A Special Case
Periods 6 and 7 are unique because of the inclusion of the lanthanides (rare earth elements) and actinides. These elements, with their f-block electron configurations, are placed separately below the main body of the periodic table to maintain a manageable table width. They are still considered part of periods 6 and 7, however, based on their electron shell arrangements and chemical properties, albeit with some nuances.
The lanthanides and actinides exhibit unique properties due to the poor shielding effect of the f-orbitals. This leads to subtle changes in atomic and ionic radii, which, consequently, impacts their chemical behavior. The f-block electrons are not as effectively shielded from the nucleus as those in the s, p, or d orbitals, resulting in a more complex interplay of forces affecting their reactivity and other properties.
Periods and Chemical Reactivity: A Closer Look
The position of an element within a period significantly influences its reactivity. Elements on the far left (alkali metals) readily lose one electron to achieve a stable electron configuration, making them highly reactive. On the opposite end, the halogens (Group 17) readily gain one electron to achieve a stable configuration, also exhibiting high reactivity. Noble gases (Group 18) possess a stable, full electron shell and thus are exceptionally unreactive.
This reactivity pattern is consistent across periods, though the degree of reactivity may vary depending on the specific element's position and electronic structure. The inner transition elements (lanthanides and actinides) exhibit a more complex reactivity pattern due to the intricacies of their f-electron configurations.
Beyond the Basics: Applications of Periodicity Understanding
Understanding the concept of periods and their implications extends beyond the theoretical. It's crucial in various fields, including:
-
Material Science: Designing new materials with specific properties often relies on the periodic trends. Understanding how the properties change across a period helps scientists tailor the composition of materials for desired functionalities (e.g., conductivity, strength, reactivity).
-
Catalysis: The catalytic activity of many elements is directly related to their position in the periodic table and subsequent electronic configuration. Knowing the trends allows scientists to predict and optimize the performance of catalysts in chemical reactions.
-
Medicine and Biology: The biological roles of many elements, such as iron, zinc, and calcium, are directly linked to their chemical properties determined by their positions within the periodic table. Understanding these trends is crucial in diagnosing and treating mineral deficiencies and designing new drugs.
-
Environmental Science: Understanding the reactivity of elements helps in predicting their environmental fate and behavior. For example, understanding the reactivity of heavy metals is critical in addressing environmental pollution and remediation strategies.
Conclusion: The Enduring Importance of Periods in Chemistry
In conclusion, the horizontal rows on the periodic table, known as periods, are far more than just a convenient organizational tool. They represent a fundamental aspect of atomic structure and are directly linked to the recurring chemical properties of elements. Understanding the trends in atomic radius, ionization energy, electronegativity, and metallic character across periods provides a crucial framework for understanding chemical reactivity, predicting material properties, and making advancements in various scientific and technological fields. The arrangement of elements into periods is a testament to the elegance and power of the periodic table as a tool for organizing and interpreting the vast world of chemical elements. The concept of periods serves as a foundational pillar of chemistry and continues to hold significant relevance in ongoing scientific exploration and technological innovation.
Latest Posts
Latest Posts
-
How To Calculate The Number Of Electrons
Mar 26, 2025
-
What Is The Least Common Multiple Of 14 And 21
Mar 26, 2025
-
What Is 10 Minutes In Hours
Mar 26, 2025
-
What Is The Greatest Common Factor Of 12 And 15
Mar 26, 2025
-
What Is Oxidized And Reduced In Cellular Respiration
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Are Horizontal Rows On The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
