How To Calculate The Number Of Electrons
listenit
Mar 26, 2025 · 5 min read
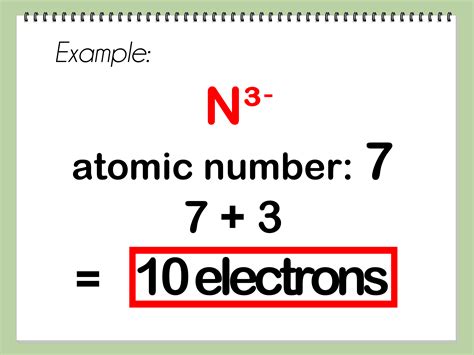
Table of Contents
- How To Calculate The Number Of Electrons
- Table of Contents
- How to Calculate the Number of Electrons: A Comprehensive Guide
- Understanding Atomic Structure and Electron Configuration
- Calculating Electrons in Neutral Atoms
- Electron Configuration and Orbital Filling
- Calculating Electrons in Ions
- Calculating Electrons in Molecules
- Advanced Considerations: Isotopes and Nuclear Reactions
- Practical Applications and Importance
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
How to Calculate the Number of Electrons: A Comprehensive Guide
Calculating the number of electrons in an atom, ion, or molecule is a fundamental concept in chemistry and physics. Understanding this allows us to predict chemical behavior, understand bonding, and analyze various physical properties. This comprehensive guide will walk you through different methods for calculating the number of electrons, covering atoms, ions, and molecules, with examples to solidify your understanding.
Understanding Atomic Structure and Electron Configuration
Before diving into calculations, let's review the basics. Atoms consist of three subatomic particles: protons, neutrons, and electrons.
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's atomic number (Z).
- Neutrons: Neutral particles (no charge) also found in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
The atomic number (Z) is crucial for electron calculation. It's found on the periodic table and represents the number of protons and, in a neutral atom, the number of electrons.
Calculating Electrons in Neutral Atoms
For a neutral atom, the number of electrons is simply equal to its atomic number.
Example 1: Calculating the number of electrons in a Carbon atom (C)
Carbon's atomic number (Z) is 6. Therefore, a neutral carbon atom has 6 electrons.
Example 2: Calculating the number of electrons in a Gold atom (Au)
Gold's atomic number is 79. A neutral gold atom has 79 electrons.
This straightforward method applies to all neutral atoms. Simply look up the atomic number on the periodic table.
Electron Configuration and Orbital Filling
While knowing the total number of electrons is important, understanding their arrangement within the atom is equally crucial for predicting chemical properties. This arrangement is described by the electron configuration. Electrons fill orbitals according to specific rules:
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy level.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons with opposite spins.
- Hund's Rule: Electrons fill orbitals individually before pairing up.
These rules help predict the electron configuration, which shows how many electrons are in each shell and subshell (s, p, d, f). For example, the electron configuration of carbon (6 electrons) is 1s²2s²2p². This indicates:
- 2 electrons in the 1s orbital
- 2 electrons in the 2s orbital
- 2 electrons in the 2p orbital
Understanding electron configuration helps visualize the atom's structure and its potential for bonding. More complex atoms require a deeper understanding of quantum mechanics to accurately determine their electron configurations. However, for many purposes, simplified versions are sufficient.
Calculating Electrons in Ions
Ions are atoms that have gained or lost electrons, resulting in a net positive (cation) or negative (anion) charge. Calculating the number of electrons in ions requires considering the charge.
- Cations: Positively charged ions formed by losing electrons. The number of electrons is the atomic number minus the charge.
- Anions: Negatively charged ions formed by gaining electrons. The number of electrons is the atomic number plus the magnitude of the charge.
Example 3: Calculating electrons in a Calcium ion (Ca²⁺)
Calcium's atomic number is 20. Ca²⁺ has lost 2 electrons. Therefore, it has 20 - 2 = 18 electrons.
Example 4: Calculating electrons in an Oxide ion (O²⁻)
Oxygen's atomic number is 8. O²⁻ has gained 2 electrons. Therefore, it has 8 + 2 = 10 electrons.
Remember to always consider the charge when calculating the number of electrons in an ion. The magnitude of the charge indicates the number of electrons gained or lost.
Calculating Electrons in Molecules
Molecules are formed by the chemical bonding of two or more atoms. The total number of electrons in a molecule is simply the sum of the electrons from each constituent atom.
Example 5: Calculating electrons in a Water molecule (H₂O)
- Oxygen (O) has 8 electrons.
- Hydrogen (H) has 1 electron each, and there are two hydrogen atoms.
Total electrons in H₂O = 8 + 1 + 1 = 10 electrons.
Example 6: Calculating electrons in a Methane molecule (CH₄)
- Carbon (C) has 6 electrons.
- Hydrogen (H) has 1 electron each, and there are four hydrogen atoms.
Total electrons in CH₄ = 6 + 1 + 1 + 1 + 1 = 10 electrons.
For larger and more complex molecules, this additive approach remains valid. Simply sum the number of electrons from each atom in the molecule.
Advanced Considerations: Isotopes and Nuclear Reactions
-
Isotopes: Isotopes of an element have the same number of protons but different numbers of neutrons. The number of electrons in a neutral isotope remains the same as its atomic number, regardless of the number of neutrons. The chemical behavior is largely determined by the number of electrons, not neutrons.
-
Nuclear Reactions: Nuclear reactions involve changes in the nucleus, affecting the number of protons and neutrons. These reactions can alter the element's identity and significantly impact the number of electrons. For instance, beta decay changes the number of protons and therefore the number of electrons in a neutral atom. Such scenarios require a more detailed understanding of nuclear physics.
Practical Applications and Importance
Accurately calculating the number of electrons is crucial in various fields:
- Chemistry: Predicting chemical bonding, reactivity, and molecular properties.
- Physics: Understanding atomic structure, spectroscopy, and the behavior of materials.
- Materials Science: Designing new materials with specific electrical, magnetic, and optical properties.
- Medicine: Developing and understanding the behavior of radioisotopes in medical imaging and therapy.
Conclusion
Calculating the number of electrons is a fundamental skill in science. While simple for neutral atoms, understanding ions and molecules requires careful consideration of charges and the number of constituent atoms. This guide has provided a comprehensive overview of the methods and concepts, empowering you to tackle a wide range of problems and appreciate the significance of electron counting in various scientific disciplines. Remember to consult the periodic table for atomic numbers and use the principles outlined above to calculate the number of electrons accurately. With practice, you'll become proficient in this crucial aspect of atomic and molecular understanding.
Latest Posts
Latest Posts
-
What Is The Lcm Of 3 9 And 15
Mar 30, 2025
-
How To Increase Concentration Of A Solution
Mar 30, 2025
-
What Is 3 6 As A Fraction
Mar 30, 2025
-
What Are The Common Factors Of 14 And 28
Mar 30, 2025
-
What Is The Slope Of The Line Shown Below
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate The Number Of Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
