How To Increase Concentration Of A Solution
listenit
Mar 30, 2025 · 5 min read
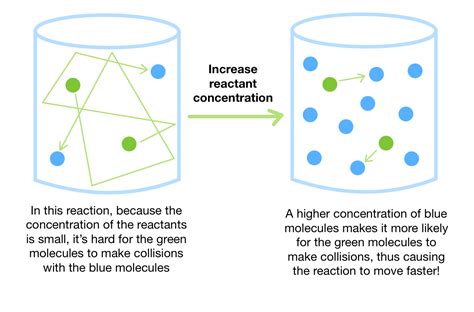
Table of Contents
How to Increase the Concentration of a Solution: A Comprehensive Guide
Increasing the concentration of a solution is a fundamental procedure in various scientific disciplines, from chemistry and biology to pharmacy and environmental science. Understanding the methods and calculations involved is crucial for accurate results and safe laboratory practices. This comprehensive guide will delve into the various techniques for increasing solution concentration, covering theoretical aspects and practical considerations.
Understanding Concentration and its Units
Before discussing the methods, it's essential to understand what concentration means. Concentration refers to the amount of solute dissolved in a given amount of solvent or solution. It's expressed using various units, the most common being:
- Molarity (M): Moles of solute per liter of solution. This is arguably the most frequently used unit in chemistry.
- Molality (m): Moles of solute per kilogram of solvent. Molality is temperature-independent, unlike molarity.
- Normality (N): Equivalents of solute per liter of solution. This unit is primarily used in acid-base and redox reactions.
- Percent concentration (%): This encompasses various forms, including:
- Weight/weight (% w/w): Grams of solute per 100 grams of solution.
- Weight/volume (% w/v): Grams of solute per 100 milliliters of solution.
- Volume/volume (% v/v): Milliliters of solute per 100 milliliters of solution.
- Parts per million (ppm) and parts per billion (ppb): These units are used for extremely dilute solutions.
Methods to Increase Solution Concentration
The primary methods for increasing solution concentration involve either adding more solute or removing solvent. The optimal method depends on the nature of the solute and solvent, the desired final concentration, and the available resources.
1. Adding More Solute
This is the most straightforward method. It involves dissolving more of the solute into the existing solution. However, several factors need consideration:
- Solubility: The solute must be soluble in the solvent. Exceeding the solubility limit will result in a saturated solution with undissolved solute. Factors influencing solubility include temperature, pressure (for gases), and the presence of other substances.
- Mixing: Thorough mixing is essential to ensure uniform distribution of the solute throughout the solution. Using a magnetic stirrer or shaking the solution vigorously can facilitate this process. For viscous solutions, a more powerful mixing method might be required.
- Heat: Increasing the temperature can often enhance the solubility of the solute, allowing for a greater amount to be dissolved. However, it's crucial to ensure that the solute and solvent are stable at the elevated temperature. Remember to allow the solution to cool to the desired temperature before final measurement or use.
- Calculation: To determine how much solute to add, you need to know the initial and desired concentrations and the volume of the solution. Detailed calculations are provided in the next section.
2. Removing Solvent
This method concentrates the solution by reducing the amount of solvent. The common techniques include:
- Evaporation: This involves heating the solution to evaporate the solvent. This is suitable for solutions where the solute is non-volatile and stable at the elevated temperature. Care must be taken to prevent bumping or splattering, which can lead to loss of solute or solution. Rotary evaporators are often used for this purpose in a lab setting.
- Distillation: Similar to evaporation, but it involves condensing and collecting the evaporated solvent. This allows for the recovery of the solvent, making it a more efficient method. It's particularly useful when separating a volatile solvent from a non-volatile solute. Different types of distillation (simple, fractional, vacuum) are available depending on the boiling points of the components.
- Freeze-drying (Lyophilization): This technique involves freezing the solution and then sublimating the ice under vacuum. This method is gentle and preserves the integrity of heat-sensitive solutes. It is often used for biological samples.
- Reverse Osmosis: This method uses pressure to force solvent molecules through a semi-permeable membrane, leaving the solute behind. This technique is effective for concentrating dilute solutions.
Calculations for Increasing Concentration
Accurate calculations are crucial for achieving the desired concentration. Let's consider an example using molarity:
Example: You have 500 mL of a 0.5 M NaCl solution and want to increase its concentration to 1.0 M.
1. Determine the moles of solute:
- Moles of NaCl initially = Molarity × Volume = 0.5 M × 0.5 L = 0.25 moles
2. Determine the desired moles of solute:
- Desired volume remains 500mL (0.5L)
- Desired moles of NaCl = Desired Molarity × Volume = 1.0 M × 0.5 L = 0.5 moles
3. Determine the additional moles of solute needed:
- Additional moles = Desired moles - Initial moles = 0.5 moles - 0.25 moles = 0.25 moles
4. Determine the mass of additional solute needed:
- Molar mass of NaCl = 58.44 g/mol
- Mass of additional NaCl = Additional moles × Molar mass = 0.25 moles × 58.44 g/mol = 14.61 g
Therefore, you need to add 14.61 g of NaCl to the 500 mL of 0.5 M solution to increase its concentration to 1.0 M. Remember to dissolve the additional NaCl completely and ensure the final volume is accurately 500 mL.
Similar calculations can be performed using other concentration units, adjusting the formulas accordingly.
Safety Precautions
Increasing the concentration of a solution can involve working with hazardous chemicals. Always adhere to these safety precautions:
- Wear appropriate personal protective equipment (PPE): This includes safety goggles, gloves, and a lab coat.
- Work in a well-ventilated area: Avoid inhaling any fumes or vapors that may be produced.
- Handle chemicals carefully: Avoid spills and skin contact.
- Follow proper disposal procedures: Dispose of waste solutions according to local regulations.
- Be aware of potential hazards: Consult the safety data sheets (SDS) for all chemicals used.
Practical Applications
The ability to adjust solution concentrations is essential across various fields:
- Chemistry: Preparing solutions for titrations, chemical reactions, and other experiments.
- Biology: Preparing culture media, buffers, and reagents for biological experiments.
- Pharmacy: Preparing medications and pharmaceutical solutions.
- Environmental Science: Analyzing water samples and preparing standard solutions for environmental testing.
- Food Science: Formulating food products and controlling the concentration of ingredients.
Conclusion
Increasing the concentration of a solution is a common laboratory procedure requiring a clear understanding of concentration units and appropriate techniques. Whether you add solute or remove solvent, careful calculations and adherence to safety protocols are crucial for achieving accurate and reliable results. Remember to always consider the properties of the solute and solvent, and choose the method best suited for your specific application. With careful planning and execution, you can confidently manipulate solution concentrations to meet the demands of your scientific endeavors.
Latest Posts
Latest Posts
-
How To Find A Quadratic Equation With Three Points
Apr 01, 2025
-
Which Form Of Electromagnetic Radiation Has The Shortest Wavelength
Apr 01, 2025
-
25 To The Power Of 1 2
Apr 01, 2025
-
How Long Is 1 4 Of A Mile
Apr 01, 2025
-
What Percent Of 35 Is 14
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How To Increase Concentration Of A Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
