Which Form Of Electromagnetic Radiation Has The Shortest Wavelength
listenit
Apr 01, 2025 · 5 min read
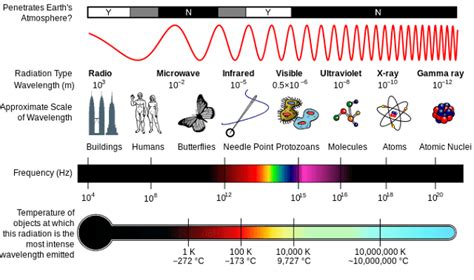
Table of Contents
Which Form of Electromagnetic Radiation Has the Shortest Wavelength?
Gamma rays hold the title for possessing the shortest wavelengths within the electromagnetic spectrum. Understanding this requires a deep dive into the nature of electromagnetic radiation and its various forms. This article will not only answer the titular question but also explore the properties, sources, and effects of gamma rays, contrasting them with other forms of electromagnetic radiation.
Understanding the Electromagnetic Spectrum
The electromagnetic spectrum is a vast range of electromagnetic radiation, encompassing all types of waves, from the longest radio waves to the shortest gamma rays. These waves are all fundamentally the same—oscillating electric and magnetic fields propagating through space—but they differ significantly in their wavelengths, frequencies, and energies. The key relationship is that wavelength and frequency are inversely proportional: shorter wavelengths correspond to higher frequencies, and vice versa. Energy is directly proportional to frequency (and inversely proportional to wavelength). This means that gamma rays, with their incredibly short wavelengths and high frequencies, also possess the highest energy.
Key Characteristics of Electromagnetic Radiation:
- Wavelength (λ): The distance between two consecutive crests (or troughs) of a wave. Measured in meters (m), nanometers (nm), Angstroms (Å), etc.
- Frequency (ν): The number of wave cycles passing a point per second. Measured in Hertz (Hz).
- Energy (E): The amount of energy carried by the wave. Related to frequency by Planck's equation: E = hν, where h is Planck's constant.
- Speed (c): The speed of light in a vacuum, approximately 3 x 10<sup>8</sup> m/s. The relationship between wavelength, frequency, and speed is: c = λν.
Gamma Rays: The High-Energy Champions
Gamma rays are the most energetic and shortest wavelength form of electromagnetic radiation. Their wavelengths are typically less than 10 picometers (10<sup>-12</sup> m), and their frequencies are incredibly high, exceeding 10<sup>19</sup> Hz. This extreme energy translates into a potent ability to interact with matter.
Sources of Gamma Rays:
Gamma rays are produced by a variety of high-energy processes in the universe:
-
Nuclear Decay: Radioactive decay of atomic nuclei is a primary source. Certain isotopes spontaneously emit gamma rays as they transition to a lower energy state. This process is used in medical imaging (nuclear medicine) and industrial applications.
-
Nuclear Fusion: The fusion reactions within stars, particularly massive stars, generate immense quantities of gamma rays. These rays are crucial to stellar energy production and transport.
-
Supernovae: The explosive deaths of massive stars release colossal bursts of gamma radiation. These supernovae are incredibly powerful events that can briefly outshine entire galaxies.
-
Neutron Stars and Pulsars: These incredibly dense stellar remnants emit beams of gamma rays that can be detected as pulsars on Earth. Their strong magnetic fields play a vital role in generating this radiation.
-
Active Galactic Nuclei (AGN): These supermassive black holes at the centers of galaxies can power jets and outflows that emit intense gamma rays.
-
Medical Applications: Gamma rays are also produced artificially in medical linear accelerators for cancer radiotherapy.
Interaction with Matter:
Due to their high energy, gamma rays interact strongly with matter, primarily through three mechanisms:
-
Photoelectric Effect: The gamma ray interacts with an atom, transferring all its energy to an electron, causing it to be ejected from the atom.
-
Compton Scattering: The gamma ray collides with an electron, transferring part of its energy and changing its direction.
-
Pair Production: At high energies, a gamma ray can interact with the electric field of an atomic nucleus, creating an electron-positron pair. The positron is the antiparticle of the electron, and when it meets an electron, they annihilate each other, releasing energy in the form of gamma rays.
Effects of Gamma Rays:
Gamma rays' high energy makes them potentially harmful to living organisms. They can ionize atoms and molecules, damaging DNA and cellular structures. High doses of gamma radiation can cause radiation sickness, cell death, and even cancer. However, controlled doses are used in radiotherapy to target and destroy cancerous cells.
Comparing Gamma Rays with Other Electromagnetic Radiation
To fully appreciate the unique nature of gamma rays, let's compare them to other parts of the electromagnetic spectrum:
X-rays:
X-rays have shorter wavelengths than ultraviolet radiation but longer wavelengths than gamma rays. They are also produced by high-energy processes, such as interactions within atoms and in astronomical objects. Medical imaging extensively uses X-rays, but their higher energy levels compared to visible light can cause damage to biological tissues with prolonged exposure. Their energy is lower than gamma rays, resulting in less potent ionization effects.
Ultraviolet (UV) Radiation:
UV radiation has wavelengths shorter than visible light but longer than X-rays. The sun is a major source of UV radiation, which can cause sunburn and contribute to skin cancer. UV radiation plays a role in certain chemical reactions and is used in sterilization techniques.
Visible Light:
Visible light is the portion of the spectrum we can see. Its wavelengths range from approximately 400 nm (violet) to 700 nm (red). Visible light is essential for vision and photosynthesis.
Infrared (IR) Radiation:
Infrared radiation has wavelengths longer than visible light. It is primarily associated with heat. We experience infrared radiation as heat, and it is used in thermal imaging and remote controls.
Microwaves:
Microwaves have even longer wavelengths than infrared radiation. They are used in cooking and communication technologies.
Radio Waves:
Radio waves have the longest wavelengths in the electromagnetic spectrum. They are used extensively in broadcasting, communication, and radar technology.
Conclusion: Gamma Rays Reign Supreme
In summary, gamma rays undoubtedly possess the shortest wavelengths of all electromagnetic radiation. Their extremely high energy, short wavelengths, and diverse sources make them a fascinating and important subject of study in physics and astronomy. While their powerful ionizing radiation necessitates cautious handling, their applications in medicine and their significance in understanding the universe are undeniable. Understanding the entire electromagnetic spectrum, from the low-energy radio waves to the high-energy gamma rays, provides a comprehensive picture of the fundamental forces shaping our universe. The properties of each form, including its wavelength, frequency, and energy, determine its applications and effects on matter, highlighting the diverse and crucial roles electromagnetic radiation plays in our lives and the cosmos.
Latest Posts
Latest Posts
-
33 As A Fraction In Simplest Form
Apr 02, 2025
-
How Many Chromosomes Does Each Daughter Cell Have
Apr 02, 2025
-
Does A Period Go Before Or After Quotations
Apr 02, 2025
-
How To Go From Vertex Form To Factored Form
Apr 02, 2025
-
Who Said I Am The State
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Form Of Electromagnetic Radiation Has The Shortest Wavelength . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
