Vsper Theory Is Used To Predict The
listenit
Mar 31, 2025 · 5 min read
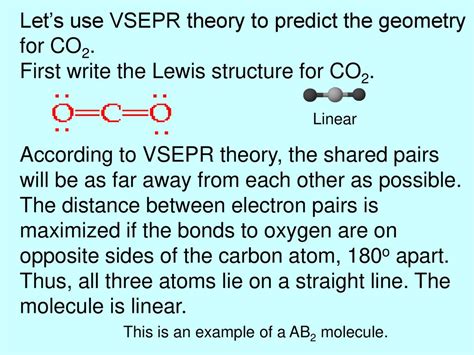
Table of Contents
VSEPR Theory: Predicting Molecular Geometry and Unveiling the Secrets of Shape
Valence Shell Electron Pair Repulsion (VSEPR) theory is a cornerstone of chemistry, providing a powerful and relatively simple model for predicting the three-dimensional shapes of molecules. Understanding VSEPR theory is crucial for comprehending the properties and reactivity of countless chemical compounds. This article delves deep into the principles of VSEPR theory, exploring its applications and limitations, and providing a comprehensive guide to predicting molecular geometries.
The Fundamental Principle: Minimizing Repulsion
At the heart of VSEPR theory lies a fundamental principle: electron pairs, whether bonding or non-bonding (lone pairs), repel each other. This repulsion is electrostatic in nature, and the electron pairs arrange themselves around the central atom in a way that minimizes this repulsion, thus determining the overall shape of the molecule. The greater the repulsion, the further apart the electron pairs will be positioned.
Types of Electron Pairs and Their Repulsive Forces
Understanding the different types of electron pairs is key to using VSEPR theory effectively:
-
Bonding Pairs (BP): These electron pairs are shared between the central atom and another atom, forming a covalent bond.
-
Lone Pairs (LP): These electron pairs are associated solely with the central atom and are not involved in bonding.
The repulsive forces between electron pairs differ:
- LP-LP repulsion > LP-BP repulsion > BP-BP repulsion
Lone pairs occupy a larger volume of space than bonding pairs due to their attraction to only one nucleus. Consequently, lone pair-lone pair repulsion is the strongest, followed by lone pair-bonding pair repulsion, and finally bonding pair-bonding pair repulsion. This hierarchy of repulsive forces significantly influences molecular geometry.
Predicting Molecular Geometry: A Step-by-Step Guide
Predicting the geometry of a molecule using VSEPR theory involves several steps:
-
Draw the Lewis Structure: This crucial first step establishes the number of bonding pairs and lone pairs around the central atom.
-
Determine the Steric Number: The steric number is the sum of the number of bonding pairs and lone pairs around the central atom. This number dictates the basic arrangement of electron pairs.
-
Identify the Electron Pair Geometry: Based on the steric number, the electron pair geometry can be determined. Common electron pair geometries include:
- Linear (Steric Number = 2): Electron pairs are arranged 180° apart.
- Trigonal Planar (Steric Number = 3): Electron pairs are arranged 120° apart in a plane.
- Tetrahedral (Steric Number = 4): Electron pairs are arranged 109.5° apart in a three-dimensional tetrahedron.
- Trigonal Bipyramidal (Steric Number = 5): Electron pairs are arranged in a trigonal bipyramidal shape.
- Octahedral (Steric Number = 6): Electron pairs are arranged 90° and 180° apart in an octahedron.
-
Determine the Molecular Geometry: The molecular geometry considers only the positions of the atoms, not the lone pairs. The presence of lone pairs distorts the ideal electron pair geometry, leading to different molecular shapes. For example, a molecule with a steric number of 4 and two lone pairs will have a bent molecular geometry, even though the electron pair geometry is tetrahedral.
Examples: Putting VSEPR Theory into Practice
Let's illustrate the application of VSEPR theory with some examples:
1. Methane (CH₄):
- Lewis Structure: Carbon is the central atom with four single bonds to hydrogen atoms.
- Steric Number: 4 (four bonding pairs)
- Electron Pair Geometry: Tetrahedral
- Molecular Geometry: Tetrahedral (all electron pairs are bonding pairs, so the molecular geometry is the same as the electron pair geometry)
2. Water (H₂O):
- Lewis Structure: Oxygen is the central atom with two single bonds to hydrogen atoms and two lone pairs.
- Steric Number: 4 (two bonding pairs, two lone pairs)
- Electron Pair Geometry: Tetrahedral
- Molecular Geometry: Bent (the lone pairs compress the H-O-H bond angle from 109.5° to approximately 104.5°)
3. Ammonia (NH₃):
- Lewis Structure: Nitrogen is the central atom with three single bonds to hydrogen atoms and one lone pair.
- Steric Number: 4 (three bonding pairs, one lone pair)
- Electron Pair Geometry: Tetrahedral
- Molecular Geometry: Trigonal Pyramidal (the lone pair pushes the hydrogen atoms closer together)
4. Carbon Dioxide (CO₂):
- Lewis Structure: Carbon is the central atom with two double bonds to oxygen atoms.
- Steric Number: 2 (two bonding pairs)
- Electron Pair Geometry: Linear
- Molecular Geometry: Linear
Limitations of VSEPR Theory
While VSEPR theory is remarkably effective in predicting molecular geometries, it has some limitations:
-
It doesn't account for multiple bonding: Double and triple bonds are treated the same as single bonds, even though they occupy more space. This can lead to minor inaccuracies in bond angle predictions.
-
It doesn't predict bond lengths: VSEPR focuses solely on the arrangement of atoms in space, not the distances between them.
-
It's less reliable for larger, more complex molecules: The interactions between electron pairs become more complex in large molecules, making accurate predictions challenging.
-
It doesn't account for relativistic effects: Relativistic effects, particularly significant for heavy atoms, can influence bond angles and molecular geometries, which are not considered in VSEPR theory.
Advanced Applications and Extensions
Despite its limitations, VSEPR theory remains a vital tool in chemistry. Its simplicity and effectiveness make it an excellent introductory model for understanding molecular shapes. More advanced models, incorporating quantum mechanics and computational methods, build upon the fundamental principles of VSEPR to achieve higher accuracy and predictive power for complex molecular systems.
Conclusion: VSEPR – A Powerful Predictive Tool
VSEPR theory provides a readily accessible and powerful method for predicting the three-dimensional structures of molecules. By understanding the principles of electron pair repulsion and the systematic approach to determining steric numbers and molecular geometries, chemists can gain invaluable insights into the properties and reactivity of a vast array of chemical compounds. While limitations exist, VSEPR theory remains a cornerstone of chemical education and a valuable tool for interpreting experimental data and predicting molecular behavior. Its simplicity makes it an excellent starting point for exploring the fascinating world of molecular structure and its relationship to chemical properties. Further exploration into advanced theoretical models and computational chemistry builds upon the foundation laid by VSEPR theory, leading to a deeper and more nuanced understanding of molecular structure and reactivity. The continued relevance of VSEPR underscores its enduring importance in the field of chemistry.
Latest Posts
Latest Posts
-
How Does Igneous Rock Become Metamorphic
Apr 01, 2025
-
How Does Friction Affect The Motion Of Objects
Apr 01, 2025
-
Sr Oh 2 Strong Or Weak
Apr 01, 2025
-
A Bond In Which Electrons Are Shared Unequally
Apr 01, 2025
-
Greatest Common Factor Of 32 And 36
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Vsper Theory Is Used To Predict The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
