Volume Of One Mole Gas At Stp
listenit
Mar 27, 2025 · 5 min read
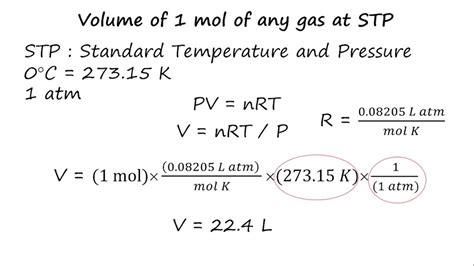
Table of Contents
The Volume of One Mole of Gas at STP: A Comprehensive Guide
The concept of the molar volume of a gas at standard temperature and pressure (STP) is a fundamental principle in chemistry, crucial for understanding gas behavior and stoichiometric calculations. This comprehensive guide will delve into the details of this concept, explaining its significance, the underlying principles, and its practical applications. We'll explore the ideal gas law, deviations from ideality, and the implications for real-world scenarios.
Understanding Standard Temperature and Pressure (STP)
Before diving into the molar volume, it's essential to define what constitutes standard temperature and pressure. For many years, STP was defined as 0° Celsius (273.15 Kelvin) and 1 atmosphere (atm) of pressure. However, the IUPAC (International Union of Pure and Applied Chemistry) has recently recommended a new standard: 0° Celsius (273.15 K) and 100 kPa (kilopascals). While both definitions are still encountered, we'll primarily focus on the newer 100 kPa standard in this article for consistency with modern scientific literature. Understanding the slight differences in pressure definitions is crucial for accurate calculations.
Key differences between the old and new STP definitions: The primary difference lies in the pressure. The older definition uses 1 atm (approximately 101.325 kPa), while the newer definition uses exactly 100 kPa. This difference leads to a slightly different molar volume calculation.
The Ideal Gas Law and Molar Volume
The behavior of ideal gases is governed by the ideal gas law:
PV = nRT
Where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of moles of the gas
- R is the ideal gas constant (8.314 J/mol·K or 0.0821 L·atm/mol·K - note the different units depending on the units of P and V)
- T is the temperature of the gas in Kelvin
This equation provides a powerful tool for determining the volume of a gas given its pressure, temperature, and number of moles. For one mole of gas (n = 1), the equation simplifies to:
PV = RT
At STP (100 kPa and 273.15 K), we can calculate the molar volume (V) using the appropriate gas constant:
Using R = 8.314 J/mol·K and converting kPa to Pa (100 kPa = 100,000 Pa):
V = (8.314 J/mol·K * 273.15 K) / (100,000 Pa) ≈ 0.0227 m³ = 22.7 L
Therefore, at STP (100 kPa, 273.15 K), the molar volume of an ideal gas is approximately 22.7 liters.
Using the older definition of STP (1 atm, 273.15 K) and R = 0.0821 L·atm/mol·K, we get:
V = (0.0821 L·atm/mol·K * 273.15 K) / (1 atm) ≈ 22.4 L
This highlights the slight difference in molar volume resulting from the differing STP definitions.
Deviations from Ideal Gas Behavior: Real Gases
It's crucial to remember that the ideal gas law is a simplification. Real gases deviate from ideal behavior, particularly at high pressures and low temperatures. These deviations arise because the ideal gas law ignores intermolecular forces (attractive and repulsive forces between gas molecules) and the volume occupied by the gas molecules themselves.
At high pressures, the gas molecules are compressed closer together, and intermolecular forces become significant. Attractive forces reduce the pressure exerted by the gas, while repulsive forces increase it. At low temperatures, the kinetic energy of the molecules decreases, making intermolecular forces more influential. This leads to a smaller volume than predicted by the ideal gas law.
The van der Waals equation is a more sophisticated model that accounts for these deviations:
(P + a(n/V)²)(V - nb) = nRT
Where:
- a and b are van der Waals constants specific to each gas. 'a' accounts for intermolecular forces, and 'b' accounts for the volume of the gas molecules.
Applications of Molar Volume
The molar volume of a gas at STP has numerous applications in chemistry and related fields:
1. Stoichiometric Calculations:
Knowing the molar volume allows us to easily convert between moles and volume of a gas at STP. This is particularly useful in stoichiometry problems, where we need to determine the amount of reactants or products involved in a chemical reaction involving gases. For instance, if a reaction produces 'x' moles of a gas at STP, we can calculate the volume of the gas produced using the molar volume.
2. Gas Density Calculations:
The density of a gas at STP can be calculated using the molar mass (M) and the molar volume (Vm):
Density = M / Vm
This calculation assumes ideal gas behavior.
3. Determining Molar Mass of Unknown Gases:
If the mass and volume of an unknown gas at STP are known, the molar mass can be calculated:
M = Density * Vm
4. Understanding Atmospheric Composition:
The molar volume concept aids in understanding the composition of the atmosphere. By knowing the partial pressures of different gases in the air and using the ideal gas law, we can calculate the molar quantities and volumes of each gas component.
5. Industrial Gas Handling:
In various industrial processes, the handling and processing of gases at STP often require accurate volume estimations. For example, chemical engineers use molar volume calculations for designing pipelines and storage tanks for gases like methane or propane.
Conclusion: The Importance of Molar Volume
The molar volume of a gas at STP, approximately 22.7 L (using the newer STP definition) or 22.4 L (using the older definition), is a cornerstone concept in chemistry. It simplifies calculations related to gas stoichiometry, density, and molar mass determination. While the ideal gas law provides a useful approximation, remembering the limitations of this model and the deviations exhibited by real gases is essential for accurate and comprehensive understanding of gas behavior in various applications. This fundamental concept bridges the gap between macroscopic measurements (volume) and the microscopic world of moles and molecules, solidifying its importance in a wide range of scientific disciplines. Always remember to specify which STP definition you are using in your calculations to avoid ambiguity and ensure accuracy in your results. By appreciating the nuances of ideal and real gas behavior, and by consistently applying the appropriate gas laws, one gains a powerful tool for exploring the fascinating world of gases.
Latest Posts
Latest Posts
-
Least Common Multiple Of 4 6 And 9
Mar 30, 2025
-
Human Body Temperature In Kelvin Scale
Mar 30, 2025
-
Which Missing Item Would Complete This Alpha Decay Reaction
Mar 30, 2025
-
Least Common Multiple Of 7 And 10
Mar 30, 2025
-
5 6 To The Power Of 2
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Volume Of One Mole Gas At Stp . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
