The Sum Of Protons And Neutrons In An Atom
listenit
Mar 18, 2025 · 6 min read
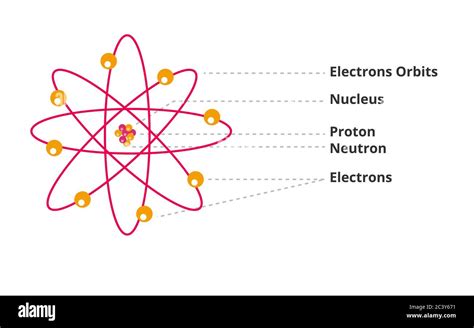
Table of Contents
The Sum of Protons and Neutrons in an Atom: A Deep Dive into Mass Number and Isotopes
The seemingly simple concept of adding protons and neutrons in an atom unlocks a deeper understanding of the fundamental building blocks of matter and the diverse properties of elements. This sum, known as the mass number, is a cornerstone of nuclear chemistry and physics, revealing crucial insights into isotopes, atomic weight, and the behavior of atoms within molecules and reactions. This article delves into the significance of this sum, exploring its implications across various scientific domains.
Understanding Protons, Neutrons, and Electrons
Before delving into the sum of protons and neutrons, let's establish a firm grasp of the fundamental subatomic particles:
Protons: The Positive Charge Carriers
Protons reside within the atom's nucleus and carry a single positive electrical charge (+1). Critically, the number of protons defines the element. An atom with one proton is hydrogen; two protons, helium; and so on. This number is known as the atomic number and is represented by the symbol Z.
Neutrons: The Neutral Partners
Neutrons, also located in the nucleus, are electrically neutral, possessing no charge. Unlike protons, the number of neutrons in an atom of a given element can vary.
Electrons: The Orbiting Negatives
Electrons orbit the nucleus in shells or energy levels. They possess a single negative charge (-1). In a neutral atom, the number of electrons equals the number of protons, maintaining an overall neutral charge.
Mass Number: The Sum of Protons and Neutrons
The mass number (A) is the total number of protons and neutrons in an atom's nucleus. It's a crucial characteristic because it represents the approximate atomic mass of the atom (in atomic mass units or amu). The equation is simple:
A = Z + N
Where:
- A is the mass number
- Z is the atomic number (number of protons)
- N is the number of neutrons
For example, a carbon atom with 6 protons and 6 neutrons has a mass number of 12 (6 + 6 = 12). This is often represented as ¹²C, where the superscript indicates the mass number.
Isotopes: Variations in Neutron Number
Isotopes are atoms of the same element (same atomic number, Z) that have different numbers of neutrons (N). Consequently, they possess different mass numbers (A). Since the chemical properties of an element are primarily determined by the number of electrons (which equals the number of protons), isotopes exhibit similar chemical behavior. However, their physical properties, particularly mass, can vary significantly.
Examples of Isotopes:
- Carbon-12 (¹²C): 6 protons, 6 neutrons (A = 12) – the most abundant isotope of carbon.
- Carbon-13 (¹³C): 6 protons, 7 neutrons (A = 13) – a stable isotope used in various applications like carbon dating.
- Carbon-14 (¹⁴C): 6 protons, 8 neutrons (A = 14) – a radioactive isotope used in radiocarbon dating.
The differences in neutron number impact the stability of the nucleus. While some isotopes are stable, others are radioactive, meaning their nuclei decay over time, emitting particles or energy. This decay process transforms the atom into a different element.
Atomic Weight: The Average Mass of Isotopes
The atomic weight (or atomic mass) listed on the periodic table is not the mass number of a single isotope. Instead, it represents the weighted average of the mass numbers of all naturally occurring isotopes of an element. The weighting takes into account the abundance of each isotope.
For instance, chlorine has two main isotopes: ³⁵Cl (75.77% abundance) and ³⁷Cl (24.23% abundance). The atomic weight of chlorine (approximately 35.45 amu) reflects this isotopic distribution.
Significance of Mass Number in Various Fields
The mass number holds significant importance across numerous scientific disciplines:
Nuclear Chemistry and Physics:
- Nuclear Reactions: Mass number plays a crucial role in understanding nuclear reactions, including fission and fusion, where the sum of protons and neutrons can change. Conservation of mass number (and atomic number) is a key principle in balancing nuclear equations.
- Nuclear Stability: The ratio of neutrons to protons affects nuclear stability. Isotopes with specific neutron-to-proton ratios tend to be more stable than others.
- Radioactive Decay: The mass number changes during radioactive decay processes such as alpha decay (loss of an alpha particle, which contains 2 protons and 2 neutrons), beta decay (conversion of a neutron to a proton or vice versa), and gamma decay (emission of gamma radiation without changing the number of protons or neutrons).
Analytical Chemistry:
- Mass Spectrometry: Mass spectrometry techniques are used to determine the isotopic composition of a sample. This analysis relies heavily on the mass-to-charge ratio of ions, which directly relates to the mass number.
- Isotope Ratio Mass Spectrometry (IRMS): IRMS is a powerful technique used in various fields, including environmental science, geochemistry, and food science, to determine the ratios of different isotopes of an element. This ratio provides information about the origin or processing history of the sample.
Biochemistry and Biology:
- Radioactive Tracers: Radioactive isotopes are used as tracers in biological systems. By tracking the movement and distribution of these isotopes, researchers can gain insights into metabolic processes and the fate of molecules within living organisms. The mass number helps distinguish between different isotopes used as tracers.
- Radiocarbon Dating: ¹⁴C, a radioactive isotope of carbon, is used to date organic materials up to approximately 50,000 years old. The decay rate of ¹⁴C is directly related to its mass number and half-life.
Materials Science and Engineering:
- Nuclear Fuels: The mass numbers of isotopes in nuclear fuels (e.g., uranium-235 and plutonium-239) are critical in determining their fission properties and suitability for nuclear reactors.
- Neutron Activation Analysis (NAA): NAA is a non-destructive analytical technique that utilizes neutrons to activate isotopes in a sample, making them radioactive. By analyzing the emitted radiation, researchers can determine the elemental composition of the sample based on their mass numbers.
Geology and Cosmochemistry:
- Geochronology: The decay rates of radioactive isotopes with known half-lives are used to date geological samples and determine the age of rocks and minerals. The mass numbers of the parent and daughter isotopes are crucial in these dating methods.
- Cosmochemistry: Studying the isotopic compositions of meteorites and other extraterrestrial materials provides insights into the formation and evolution of the solar system. Variations in the mass numbers of isotopes can reveal information about the processes that occurred during the formation of these materials.
Conclusion: The Mass Number – A Powerful Indicator
The sum of protons and neutrons in an atom, the mass number, is far more than a simple addition. It serves as a powerful indicator of an atom's identity, stability, and behavior within various systems. Understanding the significance of the mass number and its implications across scientific disciplines is crucial for advancing our knowledge of the fundamental building blocks of the universe and their interactions. From nuclear reactions to biological processes, the mass number continues to play a pivotal role in unlocking scientific mysteries and technological advancements. The further we delve into its applications, the more we realize its profound significance in the world around us.
Latest Posts
Latest Posts
-
Molecular Mass Of Ca No3 2
Mar 18, 2025
-
Square Root Of 3 Divided By 3
Mar 18, 2025
-
Highest Common Factor Of 9 And 12
Mar 18, 2025
-
How Long Is 120 Inches In Feet
Mar 18, 2025
-
Does Gas Have A Definite Shape
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about The Sum Of Protons And Neutrons In An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
