Does Gas Have A Definite Shape
listenit
Mar 18, 2025 · 5 min read
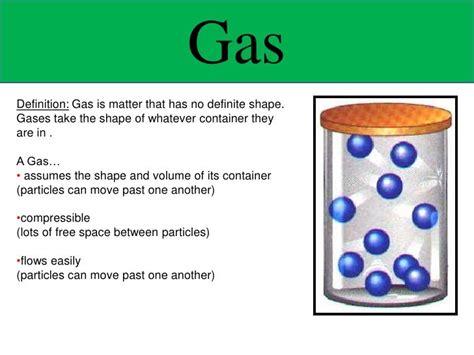
Table of Contents
Does Gas Have a Definite Shape? Exploring the Properties of Gases
The question of whether gas has a definite shape is fundamental to understanding the nature of matter. Unlike solids and liquids, gases exhibit unique properties that influence their shape and volume. This article delves into the microscopic behavior of gas molecules, explaining why gases don't possess a definite shape and exploring the concepts of compressibility, diffusion, and effusion in relation to their shapeless nature. We'll also examine how different factors, such as pressure and temperature, affect the behavior of gases.
The Kinetic Molecular Theory: The Key to Understanding Gaseous Shape
The behavior of gases is best explained by the Kinetic Molecular Theory (KMT). This theory postulates that gases consist of tiny particles (atoms or molecules) in constant, random motion. These particles are incredibly far apart compared to their size, leading to a large amount of empty space between them. This vast intermolecular distance is the primary reason why gases don't possess a definite shape.
Key Aspects of the KMT and their Impact on Shape:
-
Constant, Random Motion: Gas particles are in constant, chaotic motion, colliding with each other and the walls of their container. This constant movement prevents them from maintaining any fixed arrangement or shape. Imagine a swarm of bees – their movement is unpredictable, and they don't form a defined structure. Similarly, gas particles fill the entire available space, taking the shape of their container.
-
Negligible Intermolecular Forces: The attractive forces between gas particles are incredibly weak compared to the kinetic energy of the particles. This means the particles are essentially independent of each other, free to move and spread out without being constrained by strong intermolecular interactions. This freedom of movement directly contributes to their ability to conform to any container shape.
-
Large Intermolecular Distances: As mentioned earlier, the distance between gas particles is significantly larger than their size. This vast empty space allows the particles to move freely without significant interaction, leading to their shapeless nature. This is unlike solids, where particles are closely packed together, giving them a definite shape.
-
Elastic Collisions: Collisions between gas particles and between particles and the container walls are considered elastic. This means that there is no net loss of kinetic energy during these collisions. The constant collisions ensure the gas particles continue their random motion, further contributing to their lack of a definite shape.
Compressibility: A Defining Characteristic of Gases
One of the most striking properties of gases is their compressibility. Unlike solids and liquids, gases can be easily compressed, meaning their volume can be significantly reduced by applying pressure. This is a direct consequence of the large intermolecular distances. When pressure is applied, the particles are forced closer together, reducing the overall volume. This compressibility further emphasizes the lack of a definite shape. The gas readily adapts to the reduced volume, filling the available space, whatever shape that may be.
Diffusion and Effusion: Demonstrating the Shapeless Nature of Gases
Diffusion refers to the process where gas particles spontaneously spread out to occupy the available space. This is a direct result of the constant, random motion of the particles. If you open a bottle of perfume in a room, the scent quickly spreads throughout the room, demonstrating diffusion. This is possible because the gas particles, lacking a definite shape, easily mix with the air particles.
Effusion is a related phenomenon where gas particles escape through a tiny hole in their container. The rate of effusion depends on the mass of the gas particles; lighter particles effuse faster than heavier ones. Both diffusion and effusion highlight the lack of a defined structure in gases, illustrating their ability to spread and move freely.
Factors Affecting Gas Behavior and Shape
Several factors influence the behavior of gases, including:
-
Pressure: Increasing the pressure on a gas forces the particles closer together, reducing the volume, but it doesn't change the fundamental shapeless nature of the gas. The gas will still fill the available space, albeit a smaller one.
-
Temperature: Increasing the temperature increases the kinetic energy of the gas particles, causing them to move faster and collide more frequently. This increased motion further contributes to their ability to fill any available space, reaffirming their lack of a definite shape.
-
Volume: The volume of the container directly influences the distribution of the gas particles. Gases always expand to fill the entire available volume, regardless of the container's shape.
Ideal Gas Law and its Implications for Shape
The Ideal Gas Law, PV = nRT, relates the pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R) of a gas. This law applies best to gases at low pressure and high temperature, where intermolecular forces are negligible. The Ideal Gas Law doesn't explicitly address the shape of the gas, but it reinforces the understanding that gases will fill the available volume regardless of shape, under given conditions of pressure and temperature. The volume, V, in the equation represents the total space occupied by the gas, and the gas will fill this space irrespective of the container's shape.
Real Gases vs. Ideal Gases: Deviations from Ideal Behavior
While the Ideal Gas Law provides a good approximation for many gases under normal conditions, real gases deviate from ideal behavior at high pressures and low temperatures. At high pressures, the intermolecular forces become significant, and the volume occupied by the gas particles themselves can no longer be ignored. At low temperatures, the kinetic energy of the particles decreases, and intermolecular forces have a stronger influence. These deviations affect the gas's behavior, but they don't alter the fundamental principle that gases lack a definite shape. They simply modify the way the gas occupies the available volume.
Conclusion: Gases Are Defined by Their Lack of Shape
In conclusion, gases do not have a definite shape. Their shapelessness is a direct consequence of the fundamental properties described by the Kinetic Molecular Theory: the constant, random motion of their particles, negligible intermolecular forces, and large intermolecular distances. Factors such as pressure and temperature influence the volume and behavior of gases but do not bestow upon them a definite shape. Gases always expand to fill the entire available volume, readily adapting to the shape of their container. This intrinsic property differentiates gases from solids and liquids, emphasizing their unique physical characteristics. Understanding this fundamental property is crucial for a comprehensive understanding of chemistry and physics.
Latest Posts
Latest Posts
-
What Percent Of 50 Is 17
Mar 18, 2025
-
Write The Summary Equation For Cellular Respiration
Mar 18, 2025
-
Pauli Exclusion Principle Aufbau Principle And Hunds Rule
Mar 18, 2025
-
1 3 Divided By 1 6
Mar 18, 2025
-
Integral Of X 3 Sqrt 1 X 2
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Does Gas Have A Definite Shape . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
