Pauli Exclusion Principle Aufbau Principle And Hund's Rule
listenit
Mar 18, 2025 · 7 min read
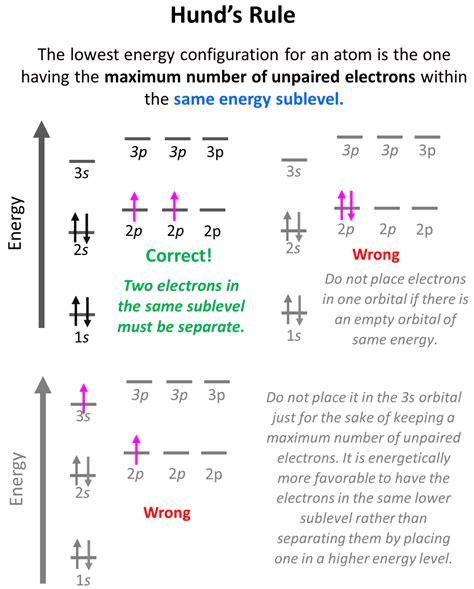
Table of Contents
Understanding Atomic Structure: Pauli Exclusion Principle, Aufbau Principle, and Hund's Rule
The seemingly simple structure of an atom belies a complex interplay of fundamental principles governing electron arrangement. Understanding atomic structure is crucial for grasping the behavior of elements and their interactions, forming the bedrock of chemistry and materials science. Three key rules—the Pauli Exclusion Principle, the Aufbau Principle, and Hund's Rule—govern how electrons populate atomic orbitals, determining an atom's properties and reactivity. This article delves into each principle, explaining their significance and illustrating their applications with examples.
The Pauli Exclusion Principle: A Fundamental Restriction
At the heart of atomic structure lies the Pauli Exclusion Principle, formulated by Wolfgang Pauli in 1925. This principle states that no two electrons in an atom can have the same set of four quantum numbers. These quantum numbers describe the unique state of an electron:
- Principal Quantum Number (n): Represents the electron shell or energy level (n = 1, 2, 3...). Higher 'n' values indicate higher energy levels and greater distance from the nucleus.
- Azimuthal Quantum Number (l): Describes the subshell or orbital shape (l = 0 to n-1). l=0 corresponds to an s orbital (spherical), l=1 to a p orbital (dumbbell-shaped), l=2 to a d orbital (more complex shapes), and so on.
- Magnetic Quantum Number (ml): Specifies the orbital orientation in space (ml = -l to +l). For example, a p subshell (l=1) has three orbitals (ml = -1, 0, +1) oriented along the x, y, and z axes.
- Spin Quantum Number (ms): Represents the intrinsic angular momentum of the electron, often visualized as a spinning motion. It can have only two values: +1/2 (spin up, ↑) or -1/2 (spin down, ↓).
The Pauli Exclusion Principle dictates that each electron within an atom must possess a unique combination of these four quantum numbers. This means that each orbital, defined by n, l, and ml, can hold a maximum of two electrons, one with spin up and one with spin down. This limitation fundamentally restricts the number of electrons that can occupy a specific energy level and subshell within an atom, directly impacting its chemical and physical behavior.
Implications of the Pauli Exclusion Principle
The Pauli Exclusion Principle is not merely an abstract rule; it has far-reaching consequences:
- Electron Configuration: It directly dictates the electron configuration of an atom, specifying how electrons are distributed among various energy levels and subshells. This configuration determines the atom's chemical reactivity and its place in the periodic table.
- Stability of Matter: The principle prevents electron collapse into the nucleus. If electrons could all occupy the lowest energy level, atoms would be incredibly unstable and matter as we know it would not exist.
- Properties of Materials: The electron configuration, governed by the Pauli Exclusion Principle, dictates the properties of materials, such as conductivity, magnetism, and color. The principle plays a crucial role in understanding the behavior of solids, liquids, and gases.
The Aufbau Principle: Building Up the Atom
The Aufbau Principle, meaning "building-up" principle in German, describes the sequential filling of atomic orbitals with electrons. Electrons fill orbitals starting from the lowest energy level and proceeding to higher energy levels. This principle is based on the increasing energy levels of orbitals:
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p ...
This order is not strictly linear due to variations in electron-electron interactions, leading to slight energy level overlaps. However, the general trend of filling orbitals from lower to higher energy remains the core of the Aufbau Principle. We can use the Aufbau Principle in conjunction with the Pauli Exclusion Principle to predict the electron configuration of any atom. For instance, let's consider the element Oxygen (O), with atomic number 8:
- Two electrons fill the 1s orbital (1s²).
- Two electrons fill the 2s orbital (2s²).
- The remaining four electrons fill the 2p orbitals (2p⁴), each electron occupying a separate 2p orbital before pairing up due to Hund's Rule (discussed below).
Therefore, the complete electron configuration of Oxygen is 1s²2s²2p⁴.
Limitations of the Aufbau Principle
While the Aufbau Principle provides a good approximation for electron configurations, it's not perfectly accurate for all elements, particularly those with partially filled d or f orbitals. Electron-electron repulsions and other factors can cause deviations from the predicted order. Exceptions often occur in the transition metals and lanthanides/actinides where energy levels of certain orbitals are very close. These exceptions highlight the limitations of a simplistic model, emphasizing the complexity of electron interactions within atoms.
Hund's Rule: Maximizing Unpaired Electrons
Hund's Rule of Maximum Multiplicity, often simply called Hund's Rule, complements the Aufbau Principle by guiding the filling of orbitals within a subshell. It states that electrons will individually occupy each orbital within a subshell before pairing up. This behavior arises from the inherent tendency of electrons to minimize electron-electron repulsion. When electrons occupy separate orbitals within a subshell, they can maintain a greater distance from each other, reducing their mutual repulsion. Each electron in a separate orbital will have the same spin (parallel spins), maximizing the total spin of the atom.
Let's revisit the Oxygen atom (O) example. The 2p subshell has three orbitals (2px, 2py, 2pz). According to Hund's Rule, the four electrons in the 2p subshell will first occupy each orbital individually before pairing up. This results in two orbitals having one electron each with parallel spins, and one orbital with a pair of electrons with opposite spins. This arrangement maximizes the total spin and minimizes electron-electron repulsion within the 2p subshell.
Hund's Rule and Magnetism
Hund's Rule is crucial for understanding the magnetic properties of atoms. Atoms with unpaired electrons exhibit paramagnetism, meaning they are attracted to a magnetic field. The more unpaired electrons, the stronger the paramagnetic behavior. Conversely, atoms with all paired electrons are diamagnetic, meaning they are slightly repelled by a magnetic field. The prediction of magnetic properties is a significant application of Hund's Rule.
The Interplay of the Three Principles
The Pauli Exclusion Principle, Aufbau Principle, and Hund's Rule work together to determine the electron configuration of any atom. The Pauli Exclusion Principle sets the fundamental limit on the number of electrons per orbital, the Aufbau Principle provides the order of filling orbitals, and Hund's Rule determines how electrons are distributed within a subshell. These three principles, in combination, are essential tools for understanding atomic structure, chemical bonding, and the properties of matter.
Applications and Further Exploration
The implications of these principles extend far beyond basic atomic structure:
- Spectroscopy: The electron configurations determined by these rules are crucial in interpreting atomic spectra, providing insights into energy level transitions and atomic properties.
- Chemical Bonding: Understanding electron configurations helps explain how atoms bond together to form molecules, influencing molecular geometry and reactivity.
- Material Science: The principles are fundamental for understanding the properties of materials, paving the way for designing new materials with specific characteristics.
- Quantum Chemistry: These rules are integrated into more complex quantum mechanical calculations used to predict molecular properties and reactions.
Further exploration into these principles might involve studying advanced concepts like Slater's rules (for more precise estimations of effective nuclear charge), relativistic effects (important for heavier elements), and the complexities of electron correlation. A deep understanding of these fundamental rules provides a strong foundation for tackling more advanced concepts in chemistry and physics.
Conclusion
The Pauli Exclusion Principle, Aufbau Principle, and Hund's Rule are cornerstone principles in atomic structure. Their elegant interplay determines the electron configuration of atoms, governing their chemical behavior and physical properties. Understanding these rules is crucial for grasping the fundamental nature of matter and its diverse manifestations in the world around us. From predicting chemical reactivity to understanding the properties of materials, these principles remain essential tools in the realm of science and technology. Continuing to explore and refine our understanding of these principles will undoubtedly unlock further insights into the complexities of the atomic world.
Latest Posts
Latest Posts
-
Can A Element Be Broken Down
Mar 19, 2025
-
Calculating The Ph At The Equivalence Point
Mar 19, 2025
-
What Is The Equivalent Fraction For 3 4
Mar 19, 2025
-
What Does A High Specific Heat Capacity Mean
Mar 19, 2025
-
Transfer Of Heat By Waves Is
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Pauli Exclusion Principle Aufbau Principle And Hund's Rule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
