The Diels Alder Reaction Is A Concerted Reaction. Define Concerted.
listenit
Mar 16, 2025 · 6 min read
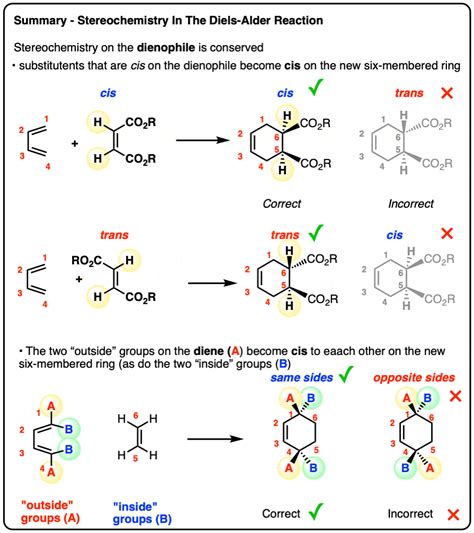
Table of Contents
The Diels-Alder Reaction: A Deep Dive into Concerted Cycloadditions
The Diels-Alder reaction, a cornerstone of organic chemistry, stands as a testament to the elegance and power of concerted reactions. Understanding its mechanism, particularly the crucial aspect of its concerted nature, is key to appreciating its synthetic utility and predicting its outcomes. This article delves into the intricacies of the Diels-Alder reaction, providing a comprehensive explanation of what "concerted" means in this context and exploring the implications of this mechanism.
What Does "Concerted" Mean in a Chemical Reaction?
Before diving into the specifics of the Diels-Alder reaction, let's define the term "concerted." In the realm of organic chemistry, a concerted reaction is a chemical transformation where bond breaking and bond formation occur simultaneously in a single, coordinated step. There is no intermediate formed during this process. All bond rearrangements happen in a synchronized manner, often via a cyclic transition state. This is in stark contrast to stepwise reactions, which involve distinct intermediates and multiple steps.
This synchronized nature of concerted reactions often leads to stereospecificity and regioselectivity, making them powerful tools for the synthesis of complex molecules with well-defined stereochemistry. The Diels-Alder reaction is a prime example of this.
The Diels-Alder Reaction: A Classic Example of a Concerted Reaction
The Diels-Alder reaction is a [4+2] cycloaddition, meaning a four-carbon atom component (the diene) reacts with a two-carbon atom component (the dienophile) to form a six-membered cyclic ring. The reaction is remarkable for its efficiency and its ability to create complex molecules with high stereoselectivity.
Key features of the Diels-Alder reaction:
- Concerted mechanism: This is the hallmark of the reaction. The formation of the two new sigma bonds and the breaking of the pi bonds in the diene and dienophile happen simultaneously.
- Stereospecificity: The stereochemistry of the reactants is directly reflected in the stereochemistry of the product. This is a direct consequence of the concerted nature of the reaction. cis dienophiles lead to cis products, and trans dienophiles lead to trans products.
- Regioselectivity: In many cases, the reaction shows regioselectivity, meaning that one regioisomer is preferentially formed over others. This is governed by factors such as the electronic effects of substituents on the diene and dienophile.
- Pericyclic Reaction: The Diels-Alder reaction is a pericyclic reaction, meaning it involves a cyclic transition state with a continuous overlap of p-orbitals. This cyclic transition state is crucial for the concerted nature of the reaction.
The Concerted Mechanism: A Detailed Look at the Transition State
The concerted nature of the Diels-Alder reaction can be best understood by examining its transition state. The reaction proceeds through a cyclic transition state where the diene adopts an s-cis conformation. This conformation allows for the simultaneous overlap of the four pi electrons of the diene and the two pi electrons of the dienophile. This overlap leads to the formation of two new sigma bonds and the breaking of the pi bonds in a single, concerted step.
Visualizing the Transition State: Imagine the diene and dienophile approaching each other. As they come closer, the p-orbitals of the diene and dienophile begin to overlap. This overlap develops into a continuous ring of overlapping p-orbitals. The electron density shifts smoothly, forming the new sigma bonds while simultaneously breaking the pi bonds. This entire process happens simultaneously, without the formation of any intermediates.
The importance of orbital symmetry: The success of the Diels-Alder reaction relies on the constructive overlap of the frontier molecular orbitals (FMOs) of the diene and dienophile. Specifically, the highest occupied molecular orbital (HOMO) of the diene interacts with the lowest unoccupied molecular orbital (LUMO) of the dienophile. This interaction leads to a favorable stabilization of the transition state, facilitating the concerted reaction.
Factors Influencing the Diels-Alder Reaction
Several factors can influence the rate and selectivity of the Diels-Alder reaction:
- Electron-withdrawing groups (EWGs) on the dienophile: EWGs on the dienophile lower the energy of its LUMO, making it more reactive towards the diene's HOMO. This generally accelerates the reaction.
- Electron-donating groups (EDGs) on the diene: EDGs on the diene raise the energy of its HOMO, enhancing its interaction with the dienophile's LUMO. This also accelerates the reaction.
- Stereochemistry of the diene and dienophile: The stereochemistry of the reactants directly influences the stereochemistry of the product, as mentioned earlier.
- Solvent effects: The solvent can influence the reaction rate and selectivity. Polar solvents tend to stabilize the polar transition state, thus accelerating the reaction.
- Temperature: Higher temperatures generally increase the reaction rate.
Distinguishing Concerted from Stepwise Reactions
It is crucial to differentiate concerted reactions from stepwise reactions. While both lead to the same overall transformation, their mechanisms are vastly different. A stepwise reaction involves the formation of discrete intermediates, each with its own distinct structure and energy. These intermediates can be isolated or observed spectroscopically.
The Diels-Alder reaction, however, leaves no such intermediate. The transformation occurs in a single, concerted step, with no detectable intermediate structures. This is a defining feature that distinguishes it from many other cycloaddition reactions that may proceed via stepwise mechanisms. Careful kinetic and spectroscopic studies have consistently supported the concerted nature of the Diels-Alder reaction.
Applications of the Diels-Alder Reaction
The Diels-Alder reaction is a powerful synthetic tool used extensively in various fields, including:
- Natural product synthesis: The reaction has played a crucial role in the synthesis of numerous natural products, owing to its ability to form complex cyclic structures with high stereoselectivity.
- Polymer chemistry: The Diels-Alder reaction is employed in the synthesis of polymers with specific properties, providing control over the polymer architecture.
- Medicinal chemistry: The reaction is instrumental in the synthesis of many pharmaceuticals, enabling the construction of crucial cyclic motifs found in biologically active molecules.
- Materials science: The reaction is used in the synthesis of materials with tailored properties, for example, in the construction of advanced materials with specific optical or electronic properties.
Beyond the Basics: Inverse Electron Demand Diels-Alder Reactions
While the typical Diels-Alder reaction involves a diene with a high HOMO and a dienophile with a low LUMO, an inverse electron demand Diels-Alder reaction involves a diene with a low HOMO and a dienophile with a high LUMO. This type of reaction is often facilitated by electron-withdrawing groups on the diene and electron-donating groups on the dienophile. Despite the difference in electronic demand, the reaction still proceeds through a concerted mechanism, emphasizing the versatility of this powerful transformation.
Conclusion: The Enduring Significance of Concerted Reactions
The Diels-Alder reaction exemplifies the power and elegance of concerted reactions in organic chemistry. Its concerted mechanism, characterized by the simultaneous bond formation and bond breaking in a single step, results in high stereoselectivity and regioselectivity, making it an indispensable tool in organic synthesis. Understanding the concerted nature of this reaction is essential for predicting reaction outcomes, designing efficient syntheses, and appreciating its profound impact on various fields of chemistry and materials science. The continued exploration and development of concerted reactions, including the Diels-Alder reaction and its variants, will undoubtedly continue to shape the future of chemical synthesis and materials design. The simplicity of its mechanism belies the profound complexity and utility of this remarkable reaction. The concerted nature is not merely an interesting observation; it is the fundamental principle underpinning its success and widespread application.
Latest Posts
Latest Posts
-
Does A Gas Have Definite Volume
Mar 16, 2025
-
What Is The Equivalent To 3 4
Mar 16, 2025
-
An Ion Is An Atom That Has
Mar 16, 2025
-
What Is 1 6 Of 24
Mar 16, 2025
-
B 13 3b 13 8 13
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about The Diels Alder Reaction Is A Concerted Reaction. Define Concerted. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
