An Ion Is An Atom That Has
listenit
Mar 16, 2025 · 6 min read
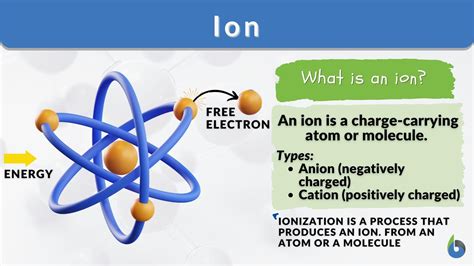
Table of Contents
An Ion is an Atom That Has… a Charge! Understanding Ionic Bonds and Their Importance
An ion is an atom or molecule that has gained or lost one or more electrons, resulting in a net electrical charge. This seemingly simple definition opens the door to a fascinating world of chemistry, influencing everything from the structure of salt crystals to the intricate processes of life itself. Understanding what makes an ion, how they're formed, and their significance is crucial for grasping fundamental chemical concepts. Let's delve into the details.
The Fundamentals: Atoms, Electrons, and Ions
Before understanding ions, we need to revisit the basics of atomic structure. Atoms are the fundamental building blocks of matter, composed of a nucleus containing positively charged protons and neutral neutrons, surrounded by negatively charged electrons. The number of protons determines the element (e.g., hydrogen has one proton, oxygen has eight). In a neutral atom, the number of protons equals the number of electrons, resulting in a net charge of zero.
Ions are formed when this balance is disrupted. When an atom loses one or more electrons, it becomes positively charged, known as a cation. Conversely, when an atom gains one or more electrons, it becomes negatively charged, known as an anion. This charge imbalance is what defines an ion.
How Ions are Formed: The Role of Electronegativity
The process of ion formation is driven by an atom's desire to achieve a stable electron configuration, typically a full outermost electron shell (also known as the valence shell). This stability is often achieved by following the octet rule, which states that atoms tend to gain, lose, or share electrons to have eight electrons in their outermost shell. Exceptions exist, particularly for elements in the first and second rows of the periodic table.
The tendency of an atom to attract electrons in a chemical bond is called electronegativity. Highly electronegative atoms are more likely to gain electrons and form anions, while atoms with low electronegativity are more likely to lose electrons and form cations. The difference in electronegativity between atoms plays a crucial role in determining the type of bond formed (ionic or covalent).
Ionic Bonds: The Electrostatic Attraction
The force of attraction between oppositely charged ions is called an ionic bond. This electrostatic interaction is the driving force behind the formation of ionic compounds. Because of the strong attraction between the positive and negative charges, ionic compounds tend to form crystalline structures with a regular, repeating pattern of ions. This structure maximizes the electrostatic attraction and minimizes repulsion.
Consider the classic example of sodium chloride (NaCl), or common table salt. Sodium (Na) has one electron in its outermost shell, while chlorine (Cl) has seven. Sodium readily loses its single valence electron to achieve a stable octet, forming a sodium cation (Na⁺). Chlorine readily gains this electron to complete its octet, forming a chloride anion (Cl⁻). The electrostatic attraction between the positively charged sodium ion and the negatively charged chloride ion forms the ionic bond that holds the crystal lattice together.
Properties of Ionic Compounds
Ionic compounds exhibit several characteristic properties stemming from the strong electrostatic forces between their constituent ions:
- High melting and boiling points: The strong ionic bonds require significant energy to overcome, resulting in high melting and boiling points.
- Crystalline structure: Ionic compounds typically form regular, three-dimensional crystal lattices.
- Solubility in water: Many ionic compounds dissolve readily in water, a polar solvent. The polar water molecules can effectively surround and separate the ions, overcoming the electrostatic attraction.
- Conductivity when molten or dissolved: When molten or dissolved in water, ionic compounds conduct electricity. The mobile ions can carry an electric current.
- Brittleness: Ionic crystals are generally brittle. Applying stress can misalign the ions, leading to repulsion and fracture.
Examples of Ions and Ionic Compounds
Numerous elements form ions, exhibiting a wide range of charges. Here are some key examples:
- Group 1 (Alkali metals): These elements readily lose one electron to form +1 cations (e.g., Li⁺, Na⁺, K⁺).
- Group 2 (Alkaline earth metals): These elements readily lose two electrons to form +2 cations (e.g., Mg²⁺, Ca²⁺, Ba²⁺).
- Group 17 (Halogens): These elements readily gain one electron to form -1 anions (e.g., F⁻, Cl⁻, Br⁻, I⁻).
- Group 16 (Chalcogens): These elements often gain two electrons to form -2 anions (e.g., O²⁻, S²⁻).
- Transition metals: Transition metals can form cations with varying charges (e.g., Fe²⁺, Fe³⁺, Cu⁺, Cu²⁺).
Examples of Ionic Compounds:
- Sodium chloride (NaCl): Table salt, formed from Na⁺ and Cl⁻.
- Magnesium oxide (MgO): A white crystalline solid, formed from Mg²⁺ and O²⁻.
- Calcium carbonate (CaCO₃): A major component of limestone and marble, formed from Ca²⁺ and CO₃²⁻.
- Potassium iodide (KI): Used in medicine and as a dietary supplement, formed from K⁺ and I⁻.
Beyond Simple Ions: Polyatomic Ions
Not all ions are single atoms. Polyatomic ions are groups of atoms covalently bonded together that carry a net electrical charge. These act as single units in ionic compounds. Examples include:
- Nitrate (NO₃⁻): Found in fertilizers and explosives.
- Sulfate (SO₄²⁻): Found in many minerals and acids.
- Phosphate (PO₄³⁻): Essential for biological systems.
- Ammonium (NH₄⁺): A common cation in fertilizers and ammonium salts.
The presence of polyatomic ions significantly expands the complexity and diversity of ionic compounds.
The Importance of Ions in Biology and Beyond
Ions play a vital role in numerous biological processes and technological applications.
Biological Significance:
- Nerve impulses: The transmission of nerve impulses relies on the movement of ions (Na⁺, K⁺, Ca²⁺) across cell membranes.
- Muscle contraction: Muscle contraction is also regulated by ion movement, particularly Ca²⁺.
- Enzyme activity: Many enzymes require specific ions as cofactors for their activity.
- Osmosis and water balance: Ion concentrations influence osmosis and maintain proper water balance in cells and tissues.
- pH regulation: Ions such as H⁺ (protons) and OH⁻ (hydroxide ions) determine the acidity or basicity (pH) of solutions, crucial for many biological processes.
Technological Applications:
- Electrolytes in batteries: Ionic compounds serve as electrolytes in batteries, allowing the flow of current.
- Corrosion prevention: Controlling ion concentrations can help prevent corrosion of metals.
- Mineral extraction: Many minerals are ionic compounds that are extracted and used in various industries.
- Fertilizers: Many fertilizers contain ionic compounds that provide essential nutrients to plants.
- Medical applications: Many ionic compounds have medical applications, including treatment of electrolyte imbalances and providing essential minerals.
Conclusion: The Ubiquitous Influence of Ions
From the simplest salt crystal to the complex processes of life, ions play a ubiquitous and essential role. Their properties, arising from the electrostatic forces between charged particles, dictate the behavior and characteristics of a vast array of substances. Understanding ionic bonds and the nature of ions is fundamental to appreciating the intricacies of chemistry and its profound influence on the world around us. Further exploration into specific ionic compounds and their applications will reveal the even more remarkable diversity and importance of this fundamental concept.
Latest Posts
Latest Posts
-
Whats The Square Root Of 40
Mar 17, 2025
-
How To Convert Wavelength To Meters
Mar 17, 2025
-
How Many Valence Electrons In Argon
Mar 17, 2025
-
1 1 X 2 Power Series
Mar 17, 2025
-
What Percent Of 75 Is 40
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about An Ion Is An Atom That Has . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
