How To Convert Wavelength To Meters
listenit
Mar 17, 2025 · 5 min read
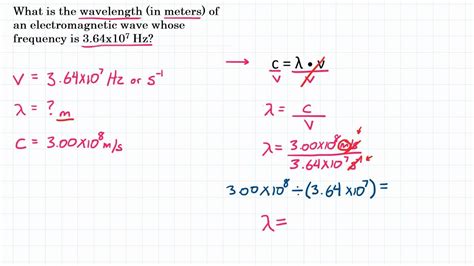
Table of Contents
How to Convert Wavelength to Meters: A Comprehensive Guide
Wavelength, a fundamental concept in physics and various scientific fields, represents the distance between consecutive corresponding points of a wave. Understanding how to convert wavelength to meters is crucial for accurate calculations and analysis in numerous applications, from optics and spectroscopy to telecommunications and astronomy. This comprehensive guide will delve into the intricacies of wavelength conversion, covering various units, formulas, and practical examples. We'll also touch upon related concepts to solidify your understanding.
Understanding Wavelength and its Units
Before we jump into the conversion process, let's establish a clear understanding of wavelength and the units commonly used to express it. Wavelength (λ, lambda) is the distance between two successive crests or troughs of a wave. It's inversely proportional to frequency (ν, nu), meaning a shorter wavelength corresponds to a higher frequency and vice-versa. This relationship is fundamental and is often expressed as:
c = λν
Where:
- c represents the speed of light (approximately 3 x 10<sup>8</sup> m/s in a vacuum).
- λ represents the wavelength.
- ν represents the frequency.
Wavelength can be expressed in various units, including:
- Meters (m): The standard unit in the International System of Units (SI).
- Nanometers (nm): Frequently used for visible light and ultraviolet (UV) radiation (1 nm = 10<sup>-9</sup> m).
- Micrometers (µm): Commonly used for infrared (IR) radiation (1 µm = 10<sup>-6</sup> m).
- Angstroms (Å): Historically used, particularly in X-ray and atomic physics (1 Å = 10<sup>-10</sup> m).
Converting Wavelength to Meters: The Core Process
The core principle behind converting wavelength from any unit to meters involves understanding the relationships between the different units. Since the meter (m) is the base unit, all conversions will ultimately involve multiplying or dividing by powers of 10.
Converting from Nanometers (nm) to Meters (m)
Nanometers are widely used in the realm of optics and spectroscopy, particularly when dealing with visible and UV light. To convert nanometers to meters, you simply need to multiply the wavelength in nanometers by 10<sup>-9</sup>.
Formula:
Wavelength (m) = Wavelength (nm) x 10<sup>-9</sup>
Example:
A laser emits light with a wavelength of 632.8 nm. To convert this to meters:
Wavelength (m) = 632.8 nm x 10<sup>-9</sup> = 6.328 x 10<sup>-7</sup> m
Converting from Micrometers (µm) to Meters (m)
Micrometers are often employed when working with infrared radiation. Similar to the nanometer conversion, converting micrometers to meters involves multiplying the wavelength in micrometers by 10<sup>-6</sup>.
Formula:
Wavelength (m) = Wavelength (µm) x 10<sup>-6</sup>
Example:
An infrared sensor detects radiation with a wavelength of 10 µm. To convert this to meters:
Wavelength (m) = 10 µm x 10<sup>-6</sup> = 1 x 10<sup>-5</sup> m
Converting from Angstroms (Å) to Meters (m)
While less common now, Angstroms are still encountered in some contexts, especially in X-ray crystallography. To convert Angstroms to meters, multiply the wavelength in Angstroms by 10<sup>-10</sup>.
Formula:
Wavelength (m) = Wavelength (Å) x 10<sup>-10</sup>
Example:
An X-ray has a wavelength of 1.54 Å. To convert this to meters:
Wavelength (m) = 1.54 Å x 10<sup>-10</sup> = 1.54 x 10<sup>-10</sup> m
Practical Applications and Examples
The conversion of wavelength to meters is essential in diverse fields:
Spectroscopy
Spectroscopy relies heavily on wavelength measurements to identify substances based on their unique absorption or emission spectra. Converting the wavelengths to meters allows for precise calculations of energy levels and other relevant parameters. For example, determining the energy of a photon requires the wavelength in meters to use the equation E = hc/λ where h is Planck's constant and c is the speed of light.
Optics and Laser Technology
In optics, understanding wavelength in meters is crucial for designing lenses, mirrors, and other optical components. The performance of lasers, widely used in various applications from barcode scanners to laser surgery, is directly linked to their wavelength. Precise wavelength determination in meters is essential for optimizing these systems.
Telecommunications
Wavelength-division multiplexing (WDM) in fiber optic communication utilizes different wavelengths of light to transmit multiple signals simultaneously along a single fiber. Converting the wavelengths to meters is essential for accurate system design and management.
Astronomy
Astronomers use wavelength measurements in meters to analyze light from celestial objects, gaining insights into their composition, temperature, and motion. Different wavelengths reveal different aspects of the universe, from the visible light we can see to the invisible radio waves and X-rays.
Advanced Concepts and Considerations
While the basic conversion process is straightforward, several advanced concepts warrant consideration:
Wavelength in Different Media
The speed of light changes when it travels through a medium other than a vacuum. This means the wavelength also changes. The relationship between the wavelength in a vacuum (λ<sub>0</sub>) and the wavelength in a medium (λ) is given by:
λ = λ<sub>0</sub> / n
where 'n' is the refractive index of the medium. Remember to use the wavelength in a vacuum for calculations involving the fundamental relationship c = λν.
Frequency and Energy Relationship
As mentioned earlier, wavelength and frequency are inversely proportional. This relationship is crucial in understanding the energy of electromagnetic radiation. The energy (E) of a photon is directly proportional to its frequency and inversely proportional to its wavelength:
E = hν = hc/λ
where 'h' is Planck's constant. Therefore, a shorter wavelength (higher frequency) corresponds to a higher energy photon.
Spectral Regions
Electromagnetic radiation spans a vast range of wavelengths, categorized into different spectral regions: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Each region has its unique characteristics and applications, and understanding the wavelength in meters within these regions is essential for effective utilization.
Conclusion
Converting wavelength to meters is a fundamental skill in many scientific and technological fields. Understanding the basic conversion processes, coupled with the knowledge of related concepts like frequency, energy, and refractive index, empowers you to perform accurate calculations and analysis. Mastering this skill opens doors to a deeper understanding of the properties of light and electromagnetic radiation, furthering your capabilities in various applications. Remember to always pay attention to the units and use the appropriate conversion factors to ensure accurate results. This comprehensive guide has provided a strong foundation; further exploration into specific fields will offer more specialized applications of wavelength conversions.
Latest Posts
Latest Posts
-
What Percentage Of 50 Is 20
Mar 17, 2025
-
Simplify The Square Root Of 10
Mar 17, 2025
-
Common Multiple Of 9 And 3
Mar 17, 2025
-
What Is The Ph Of A Neutral Solution
Mar 17, 2025
-
What Is The Number Of Valence Electrons For Nitrogen
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How To Convert Wavelength To Meters . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
