How Many Valence Electrons In Argon
listenit
Mar 17, 2025 · 6 min read
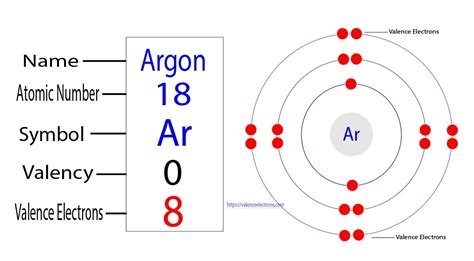
Table of Contents
How Many Valence Electrons in Argon? Understanding Noble Gas Configuration
Argon, a fascinating noble gas, holds a unique position in the periodic table. Its stability and electronic structure are key to understanding its chemical behavior and properties. A frequently asked question, particularly in chemistry classes, revolves around the number of valence electrons it possesses. This comprehensive guide delves into the answer, explaining the concept of valence electrons, argon's electron configuration, and the implications of its full valence shell.
Understanding Valence Electrons: The Outer Shell Players
Before diving into argon's specific electron count, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are crucial because they are the ones primarily involved in chemical bonding. They determine how an atom will interact with other atoms, whether it will readily form bonds, and the type of bonds it will form (ionic, covalent, metallic). The number of valence electrons directly influences an element's chemical reactivity and properties.
Identifying Valence Electrons: A Quick Guide
The number of valence electrons can generally be determined by an element's position within the periodic table. For the main group elements (Groups 1-18), the group number (using the older numbering system) corresponds directly to the number of valence electrons. For example, elements in Group 1 (alkali metals) have one valence electron, Group 2 (alkaline earth metals) have two, and so on. However, this rule doesn't apply to transition metals or inner transition metals (lanthanides and actinides) due to the complex filling of their d and f orbitals.
Argon's Electronic Structure: A Noble Gas Configuration
Argon (Ar), with an atomic number of 18, has 18 electrons orbiting its nucleus. To understand its valence electron count, we need to examine its electron configuration. The electron configuration describes how electrons are distributed among different energy levels and sublevels within an atom. Argon's electron configuration is 1s²2s²2p⁶3s²3p⁶.
Decoding Argon's Electron Configuration
Let's break down Argon's electron configuration:
- 1s²: Two electrons occupy the first energy level (n=1), in the 's' subshell.
- 2s²: Two electrons occupy the second energy level (n=2), in the 's' subshell.
- 2p⁶: Six electrons occupy the second energy level (n=2), in the 'p' subshell.
- 3s²: Two electrons occupy the third energy level (n=3), in the 's' subshell.
- 3p⁶: Six electrons occupy the third energy level (n=3), in the 'p' subshell.
The outermost shell for argon is the third energy level (n=3). This shell contains the 3s and 3p sublevels, and they are completely filled with a total of eight electrons (2 + 6 = 8).
The Answer: Argon's Eight Valence Electrons
Therefore, Argon has eight valence electrons. This full valence shell of eight electrons (an octet) is the reason for argon's exceptional stability and its inert nature.
The Significance of a Full Valence Shell
A full valence shell signifies a particularly stable electronic configuration. Atoms tend to gain, lose, or share electrons to achieve a full valence shell, mimicking the stable electron arrangement of noble gases. This drive for stability is the fundamental principle underlying chemical bonding. Since argon already possesses a complete octet, it has little tendency to participate in chemical reactions or form bonds with other atoms. This is why argon is considered a noble gas – it's chemically unreactive under normal conditions.
Argon's Properties and Applications: A Consequence of its Valence Electrons
Argon's unique properties, directly stemming from its eight valence electrons and resultant chemical inertness, lead to numerous applications across various fields:
1. Inert Atmosphere: Protecting Reactions and Materials
Argon's lack of reactivity makes it ideal for creating inert atmospheres in various industrial processes. It is used in:
- Welding: Argon shields the weld pool from atmospheric oxygen and nitrogen, preventing oxidation and contamination, leading to stronger and higher-quality welds.
- Metal Production: Protecting molten metals during manufacturing prevents unwanted reactions with the atmosphere.
- Chemical Reactions: Argon provides an inert environment for sensitive chemical reactions, preventing unwanted side reactions.
- Sample Preservation: Argon is used to store and preserve samples that are sensitive to oxidation or moisture.
2. Lighting Applications: The Glow of Argon
Argon is utilized in:
- Fluorescent Lights: It enhances the performance and longevity of fluorescent tubes by preventing the filament from burning out quickly and improving the light emission.
- Incandescent Lights: Argon creates an inert atmosphere, preventing the filament from oxidation and extending its lifespan.
- Neon Lights (sometimes): While neon lights are famous, argon is also used, often in combination with other gases, to create different colors of light.
3. Medical Applications: Protecting and Imaging
Argon finds applications in:
- Laser Surgery: Argon lasers are used in various medical procedures, including eye surgery, due to their precise and controllable nature.
- Cryosurgery: Argon is sometimes used in cryosurgery, where extremely low temperatures are applied to destroy unwanted tissues.
4. Other Applications
Argon's versatile nature also extends to other applications, including:
- Dating Techniques: Argon-argon dating, a radiometric dating method, is used to determine the age of geological samples.
- Refrigeration: Argon is employed as a refrigerant in some specialized applications.
Beyond the Basics: Exploring Deeper into Argon's Electronic Structure
While understanding the eight valence electrons is fundamental, a more nuanced examination of Argon's electron configuration can provide deeper insights into its properties.
Orbital Filling and Energy Levels
The electron configuration reveals the order in which electrons fill the atomic orbitals. Electrons initially occupy lower energy levels before moving to higher ones. The Pauli Exclusion Principle dictates that each orbital can hold a maximum of two electrons with opposite spins. Hund's Rule governs the filling of orbitals within a subshell, stating that electrons will occupy orbitals individually before pairing up. Understanding these rules is key to comprehending the intricacies of electron configuration and atomic structure.
Ionization Energy and Electron Affinity
Argon's high ionization energy – the energy required to remove an electron from an atom – is another consequence of its stable, full valence shell. It requires a significant amount of energy to remove an electron from the stable octet. Similarly, Argon has a very low electron affinity, the energy change associated with adding an electron to a neutral atom, because adding an electron would disrupt its stable configuration.
Conclusion: Argon's Significance in Chemistry and Beyond
Argon, with its eight valence electrons and resulting chemical inertness, plays a crucial role in numerous applications, ranging from industrial processes to medical procedures. Understanding its electronic structure, particularly the significance of its complete valence shell, is essential for appreciating its unique properties and diverse applications. The seemingly simple answer – eight valence electrons – unlocks a deeper understanding of Argon's fundamental chemical behavior and its indispensable role in our modern world. From welding to lighting to medical advancements, argon's impact is significant and continues to grow. The exploration of its properties underscores the vital connection between fundamental atomic structure and the macroscopic world.
Latest Posts
Latest Posts
-
What Percent Of 40 Is 75
Mar 17, 2025
-
Do Quotes Go Before Or After The Period
Mar 17, 2025
-
What Is The Molar Mass Of Nh4 2co3
Mar 17, 2025
-
What Percentage Of 50 Is 20
Mar 17, 2025
-
Simplify The Square Root Of 10
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Argon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
