Does A Gas Have Definite Volume
listenit
Mar 16, 2025 · 5 min read
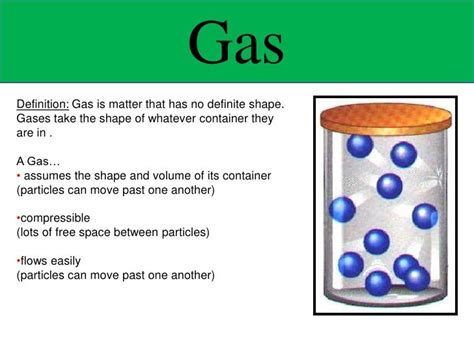
Table of Contents
- Does A Gas Have Definite Volume
- Table of Contents
- Does a Gas Have a Definite Volume? Understanding the Behavior of Gases
- Understanding the Kinetic Molecular Theory of Gases
- The Ideal Gas Law: A Mathematical Representation
- Real Gases vs. Ideal Gases: Deviations from Ideal Behavior
- Comparing Gases, Liquids, and Solids: A Contrast in Volume
- Solids: Definite Volume and Shape
- Liquids: Definite Volume, Indefinite Shape
- Gases: Indefinite Volume and Shape
- Practical Implications of Indefinite Gas Volume
- Conclusion: A Dynamic and Adaptable State of Matter
- Latest Posts
- Latest Posts
- Related Post
Does a Gas Have a Definite Volume? Understanding the Behavior of Gases
The question of whether a gas has a definite volume is a fundamental concept in chemistry and physics. The simple answer is no, a gas does not have a definite volume. Unlike solids and liquids, which maintain a relatively fixed shape and volume, gases are highly compressible and readily expand or contract to fill their container. This behavior is governed by the principles of kinetic molecular theory and the ideal gas law. This article delves deeper into this concept, exploring the factors that influence gas volume, the differences between gases, liquids, and solids, and the practical implications of this characteristic.
Understanding the Kinetic Molecular Theory of Gases
To understand why gases don't have a definite volume, we need to examine the kinetic molecular theory (KMT). This theory postulates that gases consist of tiny particles (atoms or molecules) that are in constant, random motion. These particles are widely separated compared to their size, resulting in a lot of empty space. The key characteristics of gases according to KMT are:
- Constant, Random Motion: Gas particles are constantly moving in straight lines until they collide with each other or the container walls.
- Negligible Intermolecular Forces: The attractive forces between gas particles are weak and negligible compared to their kinetic energy. This means particles are essentially independent of each other.
- Elastic Collisions: Collisions between gas particles and the container walls are perfectly elastic, meaning no kinetic energy is lost during the collision.
- Volume of Particles is Negligible: The volume occupied by the gas particles themselves is insignificant compared to the total volume of the container.
Because of these characteristics, gas particles are free to move and occupy the entire available space within a container. If the container is expanded, the gas will expand to fill the larger volume. Conversely, if the container is compressed, the gas will be compressed into the smaller volume. This explains why gases don't possess a fixed volume; their volume is determined entirely by the volume of the container they occupy.
The Ideal Gas Law: A Mathematical Representation
The ideal gas law is a mathematical equation that describes the behavior of ideal gases. An ideal gas is a theoretical gas that perfectly follows the postulates of the kinetic molecular theory. The ideal gas law is expressed as:
PV = nRT
Where:
- P represents pressure
- V represents volume
- n represents the number of moles of gas
- R represents the ideal gas constant
- T represents temperature
This equation shows that volume (V) is directly proportional to the number of moles (n) and temperature (T), and inversely proportional to pressure (P). This means that if you increase the number of gas particles (n) or the temperature (T), the volume will increase. Conversely, if you increase the pressure (P), the volume will decrease. This relationship clearly demonstrates that the volume of a gas is not inherent to the gas itself but is dependent on external factors.
Real Gases vs. Ideal Gases: Deviations from Ideal Behavior
It's important to note that the ideal gas law is a simplification. Real gases don't always behave perfectly according to the ideal gas law, especially under conditions of high pressure and low temperature. At high pressures, the volume occupied by the gas particles themselves becomes significant, and intermolecular forces become more pronounced. These deviations from ideal behavior lead to slight variations in the relationship between pressure, volume, temperature, and the number of moles. However, the fundamental principle remains: the volume of a gas is not fixed but rather depends on the conditions it is subjected to.
Comparing Gases, Liquids, and Solids: A Contrast in Volume
To further illustrate the concept of indefinite volume in gases, let's compare them to liquids and solids:
Solids: Definite Volume and Shape
Solids possess a definite volume and shape. Their particles are tightly packed together in a fixed arrangement, resulting in strong intermolecular forces. This structure prevents them from easily compressing or expanding, thus maintaining a constant volume regardless of the container they are in.
Liquids: Definite Volume, Indefinite Shape
Liquids have a definite volume but an indefinite shape. Their particles are closer together than in gases but still have some freedom of movement. They can flow and take the shape of their container, but their volume remains relatively constant.
Gases: Indefinite Volume and Shape
As discussed previously, gases have both indefinite volume and shape. Their particles are far apart, with weak intermolecular forces, allowing them to readily expand or contract to fill the available space.
This comparison highlights the unique characteristic of gases: their ability to adapt their volume to the container's volume, unlike solids and liquids.
Practical Implications of Indefinite Gas Volume
The indefinite volume of gases has numerous practical implications across various fields:
- Pneumatics and Hydraulics: Pneumatic systems utilize compressed gases to power machinery and tools. The compressibility of gases is crucial for these systems to function efficiently.
- Weather Balloons: Weather balloons expand as they ascend to higher altitudes where the atmospheric pressure is lower. The change in volume is directly related to the change in pressure.
- Internal Combustion Engines: The expansion and compression of gases within internal combustion engines are critical to their operation. The volume change drives the pistons and generates power.
- Aerosol Cans: Aerosol cans utilize the pressure of a compressed gas to propel the contents. The volume of the gas changes as the contents are dispensed.
- Breathing and Respiration: Our lungs expand and contract to take in and expel air, demonstrating the compressibility and variable volume of gases in a biological context.
Conclusion: A Dynamic and Adaptable State of Matter
In conclusion, a gas does not possess a definite volume. Its volume is determined by the container it occupies and is influenced by pressure and temperature. This characteristic stems from the kinetic molecular theory, which describes the constant motion and negligible intermolecular forces of gas particles. The ideal gas law provides a mathematical framework for understanding this behavior, although real gases can deviate from ideal behavior under certain conditions. The indefinite volume of gases has far-reaching implications across various scientific and technological applications. Understanding this fundamental property is essential for comprehending the behavior of gases and their interactions with the world around us. The unique properties of gases, including their compressibility and ability to fill containers, set them apart from liquids and solids and are crucial to a vast number of natural processes and technological advancements.
Latest Posts
Latest Posts
-
How Many Valence Electrons In Argon
Mar 17, 2025
-
1 1 X 2 Power Series
Mar 17, 2025
-
What Percent Of 75 Is 40
Mar 17, 2025
-
Translating Graph Up By 4 Units
Mar 17, 2025
-
What Is The Least Common Multiple Of 3 2
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Does A Gas Have Definite Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
