Substance That Speeds Up The Rate Of A Chemical Reaction
listenit
Mar 25, 2025 · 6 min read
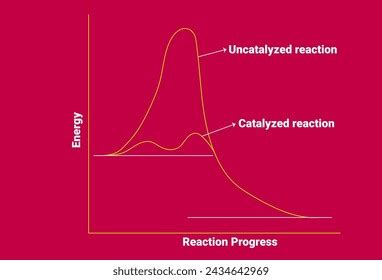
Table of Contents
Substances That Speed Up the Rate of a Chemical Reaction: A Deep Dive into Catalysts
Chemical reactions are the foundation of our world, driving everything from digestion in our bodies to the manufacturing of plastics. The speed at which these reactions occur is crucial, and often, we need to manipulate this speed to achieve desired outcomes. This is where catalysts come in – substances that dramatically increase the rate of a chemical reaction without being consumed in the process. This article explores the fascinating world of catalysts, delving into their mechanisms, types, applications, and importance across various fields.
Understanding Chemical Reaction Rates
Before we dive into catalysts, it's crucial to understand what influences the rate of a chemical reaction. Several factors play a significant role:
-
Concentration of reactants: Higher concentrations generally lead to faster reactions, as there are more reactant molecules available to collide and react.
-
Temperature: Increasing the temperature provides reactant molecules with more kinetic energy, leading to more frequent and energetic collisions, thus accelerating the reaction rate.
-
Surface area: For reactions involving solids, a larger surface area exposes more reactant molecules to interaction, enhancing the reaction rate.
-
Presence of a catalyst: As we'll explore in detail, catalysts significantly alter the reaction pathway, lowering the activation energy and thus accelerating the reaction.
What are Catalysts?
A catalyst is a substance that increases the rate of a chemical reaction without itself being consumed in the process. It achieves this by providing an alternative reaction pathway with a lower activation energy. The activation energy is the minimum energy required for reactants to transform into products. By lowering this energy barrier, the catalyst enables a greater proportion of reactant molecules to successfully overcome the energy hurdle and proceed to form products.
How Catalysts Work: The Mechanism
Catalysts function by forming temporary bonds with reactant molecules, creating an intermediate complex. This complex then undergoes transformations that ultimately lead to the formation of products and the regeneration of the catalyst. This process can involve several steps:
-
Adsorption: Reactant molecules adsorb onto the catalyst's surface, forming a temporary bond.
-
Activation: The adsorbed molecules undergo changes in their electronic structure, weakening existing bonds and facilitating the formation of new ones.
-
Reaction: The activated molecules react to form products.
-
Desorption: The products desorb from the catalyst's surface, leaving the catalyst unchanged and ready to catalyze further reactions.
This mechanism highlights the crucial role of the catalyst's surface area. A larger surface area provides more active sites for reactant adsorption and reaction, increasing the overall catalytic efficiency.
Types of Catalysts
Catalysts can be broadly classified into two main categories:
1. Homogeneous Catalysts
Homogeneous catalysts exist in the same phase (gas or liquid) as the reactants. They are often dissolved in the reaction mixture and participate directly in the reaction mechanism. Examples include acid-catalyzed esterification reactions where a strong acid like sulfuric acid acts as a homogeneous catalyst. The acid protonates the carbonyl group of the carboxylic acid, making it more susceptible to nucleophilic attack by the alcohol.
2. Heterogeneous Catalysts
Heterogeneous catalysts exist in a different phase from the reactants. Typically, they are solids, while the reactants are gases or liquids. The reaction occurs on the surface of the heterogeneous catalyst. A classic example is the Haber-Bosch process for ammonia synthesis, where a finely divided iron catalyst facilitates the reaction between nitrogen and hydrogen gases. The high surface area of the iron catalyst is critical for its effectiveness.
Enzyme Catalysts: Nature's Master Catalysts
Enzymes are biological catalysts, primarily proteins, that accelerate biochemical reactions within living organisms. They exhibit remarkable specificity and efficiency, often exceeding the catalytic power of synthetic catalysts. Enzymes achieve this through their intricate three-dimensional structures, which create active sites specifically designed to bind to substrates (reactant molecules) and facilitate their transformation into products. The lock-and-key model and the induced-fit model describe how enzymes interact with their substrates.
Applications of Catalysts
Catalysts are ubiquitous and play a vital role across a wide range of industries and processes:
1. Industrial Processes:
-
Petroleum refining: Catalysts are essential for cracking heavier hydrocarbons into lighter, more valuable products like gasoline.
-
Polymer production: Catalysts are employed in the synthesis of numerous polymers, including plastics, rubbers, and fibers.
-
Chemical manufacturing: Catalysts are crucial for the efficient production of various chemicals, including ammonia, sulfuric acid, and methanol.
-
Automotive catalytic converters: These devices utilize catalysts to convert harmful exhaust gases into less harmful substances.
2. Environmental Applications:
-
Water purification: Catalysts are used in advanced oxidation processes to remove pollutants from water.
-
Air pollution control: Catalysts help in removing harmful pollutants from the air.
3. Biomedical Applications:
-
Drug development: Catalysts play a crucial role in the synthesis of many pharmaceuticals.
-
Medical diagnostics: Enzyme-based assays are used in many diagnostic tests.
Catalyst Selection and Design
The choice of catalyst for a specific reaction depends on several factors, including:
-
Reaction type: Different catalysts are suited for different reactions.
-
Reaction conditions: Factors like temperature, pressure, and solvent influence catalyst performance.
-
Selectivity: A catalyst should ideally promote the desired reaction pathway while minimizing the formation of unwanted byproducts.
-
Stability: The catalyst should maintain its activity and selectivity over extended periods.
-
Cost and availability: Economic considerations are also important.
Catalyst design is an active area of research, focusing on developing more efficient, selective, and sustainable catalysts. This involves tailoring the catalyst's structure and composition to optimize its performance.
The Importance of Catalysts
Catalysts are indispensable for modern society. Their ability to accelerate chemical reactions at lower temperatures and pressures leads to:
-
Increased efficiency: Catalysts reduce the energy consumption and production costs associated with chemical processes.
-
Reduced waste: Catalysts promote cleaner reactions, minimizing the formation of undesirable byproducts.
-
Sustainable development: Catalysts enable the development of greener and more environmentally friendly chemical processes.
-
Technological advancements: The development of new catalysts is crucial for advancing various technologies and industries.
Future Directions in Catalysis
Research in catalysis continues to push the boundaries of what's possible. Some key areas of focus include:
-
Development of novel catalytic materials: Scientists are exploring new materials with unique properties for catalysis. This includes the use of nanomaterials, metal-organic frameworks, and other advanced materials.
-
Computational catalysis: Computer simulations are increasingly used to design and optimize catalysts.
-
Green catalysis: Focus is on developing catalysts that are environmentally benign and use sustainable resources.
-
Biocatalysis: Further development and application of enzyme catalysis offers unique opportunities for selective and efficient transformations.
-
Heterogeneous catalysis using single-atom catalysts: These advanced catalysts offer high selectivity and efficiency.
Conclusion
Catalysts are remarkable substances that play a pivotal role in countless chemical processes, driving technological advancements and shaping our world. Their ability to accelerate reactions with remarkable efficiency and selectivity makes them essential across various industries and scientific disciplines. Continued research and development in catalysis will undoubtedly lead to further breakthroughs in materials science, medicine, and environmental remediation, shaping a future fueled by more efficient and sustainable chemical transformations. Understanding the intricacies of catalysis, its mechanisms, and applications is crucial for advancing science and technology for the benefit of humankind.
Latest Posts
Latest Posts
-
An Atom That Gains An Electron Is Called
Mar 28, 2025
-
How Many Grams In A 1 8
Mar 28, 2025
-
Simplify The Square Root Of 192
Mar 28, 2025
-
What Is The Fraction For 0 875
Mar 28, 2025
-
How Many Inches Are In One Square Foot
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Substance That Speeds Up The Rate Of A Chemical Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
