An Atom That Gains An Electron Is Called
listenit
Mar 28, 2025 · 6 min read
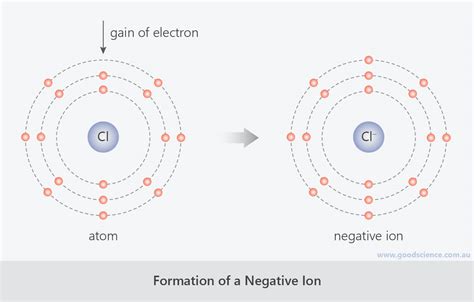
Table of Contents
An Atom That Gains an Electron is Called an Anion: A Deep Dive into Ionic Bonding and Chemical Reactions
When an atom gains an electron, it undergoes a fundamental change in its electrical charge and properties. This process is crucial in understanding chemical bonding, particularly ionic bonding, and a wide array of chemical reactions. Let's delve into the specifics, exploring the terminology, the underlying principles, and the significant implications of an atom gaining an electron.
Understanding Atomic Structure: The Foundation of Charge
Before we explore the intricacies of an atom gaining an electron, it's essential to grasp the basics of atomic structure. Atoms are the fundamental building blocks of matter, composed of a central nucleus containing protons (positively charged particles) and neutrons (neutral particles), surrounded by orbiting electrons (negatively charged particles). The number of protons determines the element's identity (its atomic number), while the number of electrons usually equals the number of protons, resulting in a neutral atom.
The Role of Electrons in Chemical Behavior
Electrons occupy specific energy levels or shells around the nucleus. The outermost shell, known as the valence shell, holds the valence electrons, which are directly involved in chemical bonding. Atoms strive for stability, typically aiming to have a full valence shell, either by gaining, losing, or sharing electrons. This drive for stability is the driving force behind countless chemical reactions.
The Transformation: When an Atom Gains an Electron
When a neutral atom gains an electron, it acquires an extra negative charge. This process transforms the atom into a negatively charged ion, specifically known as an anion. The addition of the electron upsets the balance between the positive charges of the protons and the negative charges of the electrons. The atom now possesses more electrons than protons, resulting in a net negative charge.
The Significance of Anion Formation
Anion formation has profound implications in various aspects of chemistry and beyond:
-
Ionic Bonding: Anions are essential components of ionic bonds. Ionic bonds form when a metal atom (which readily loses electrons to become a positively charged cation) and a non-metal atom (which readily gains electrons to become an anion) interact. The electrostatic attraction between the oppositely charged ions holds the compound together. Table salt (NaCl), for example, is formed through an ionic bond between a sodium cation (Na⁺) and a chloride anion (Cl⁻).
-
Chemical Reactions: Many chemical reactions involve the transfer of electrons, leading to the formation of anions. Redox reactions (oxidation-reduction reactions) are a prime example. Oxidation involves the loss of electrons (forming cations), while reduction involves the gain of electrons (forming anions). These reactions are ubiquitous in biological processes, industrial applications, and environmental chemistry.
-
Solubility and Conductivity: Anions, along with cations, influence the solubility of ionic compounds in water. The strength of the ionic bond and the interaction between the ions and water molecules determine the solubility. Furthermore, solutions of ionic compounds are typically good conductors of electricity because the freely moving ions carry electrical charge.
-
Biological Systems: Anions play crucial roles in various biological processes. For instance, chloride ions (Cl⁻) are vital for maintaining fluid balance and nerve impulse transmission. Phosphate ions (PO₄³⁻) are essential components of DNA and ATP (adenosine triphosphate), the energy currency of cells. Many other anions contribute to essential biological functions.
Understanding the Naming Conventions of Anions
The naming of anions follows systematic rules that are based on the parent element. Understanding these naming conventions is crucial for communicating clearly and accurately in the field of chemistry:
-
Monatomic Anions: These are anions derived from a single atom. They are named by adding the suffix "-ide" to the root name of the element. For example:
- Chlorine (Cl) becomes Chloride (Cl⁻)
- Oxygen (O) becomes Oxide (O²⁻)
- Sulfur (S) becomes Sulfide (S²⁻)
- Nitrogen (N) becomes Nitride (N³⁻)
-
Polyatomic Anions: These are anions composed of two or more atoms covalently bonded together. The naming conventions for polyatomic anions are more varied and often involve specific prefixes and suffixes. Some common examples include:
- Sulfate (SO₄²⁻)
- Nitrate (NO₃⁻)
- Phosphate (PO₄³⁻)
- Carbonate (CO₃²⁻)
- Hydroxide (OH⁻)
The charges of polyatomic anions vary depending on the elements and their bonding arrangements. Understanding the charges of these anions is critical for balancing chemical equations and predicting the properties of ionic compounds.
Factors Influencing Anion Formation
Several factors influence the likelihood of an atom forming an anion:
-
Electron Affinity: Electron affinity is a measure of an atom's ability to attract and accept an electron. Atoms with high electron affinities are more likely to form anions. Non-metals, particularly those in Groups 16 and 17 (chalcogens and halogens) generally exhibit high electron affinities.
-
Electronegativity: Electronegativity is a measure of an atom's ability to attract electrons within a chemical bond. Atoms with high electronegativity are more likely to attract electrons from other atoms, leading to anion formation. Again, non-metals generally have higher electronegativities than metals.
-
Ionic Radius: The size of the resulting anion also plays a role. Larger anions are generally more stable than smaller anions due to the increased distance between the electrons and the nucleus, leading to less electron-electron repulsion.
Applications and Significance of Anion Chemistry
The chemistry of anions is vast and has significant implications across numerous fields:
-
Materials Science: Anions play crucial roles in the properties of various materials. For example, the oxide anions in ceramics determine their strength and hardness, while halide anions in polymers influence their flexibility and thermal stability.
-
Environmental Chemistry: Anions are involved in numerous environmental processes. For example, nitrate anions (NO₃⁻) contribute to water pollution, while sulfate anions (SO₄²⁻) are involved in acid rain. Understanding the behavior of anions in the environment is critical for environmental remediation and protection.
-
Medicine and Biology: Anions are integral to many biological processes and medical applications. Chloride ions are essential for maintaining osmotic balance, while bicarbonate ions (HCO₃⁻) play a vital role in blood pH regulation. Many pharmaceutical drugs contain anions as active components.
-
Industrial Processes: Anions are utilized extensively in various industrial processes. For example, chloride anions are used in the production of plastics, while phosphate anions are used in fertilizers. Understanding anion chemistry is crucial for optimizing these processes and developing new technologies.
Conclusion: The Central Role of Anions in Chemistry
An atom that gains an electron is called an anion, a negatively charged ion that plays a critical role in chemical bonding, chemical reactions, and a multitude of applications. From the formation of ionic compounds and the balance of biological systems to the optimization of industrial processes and environmental remediation, the study of anions and their behavior is fundamental to understanding the world around us. This article has provided a comprehensive overview of anion formation, naming conventions, influencing factors, and applications, offering a foundational understanding for further exploration in the fascinating field of chemistry. The importance of anions extends far beyond the classroom, impacting various fields of scientific endeavor and technological advancements. A deep understanding of anion chemistry is vital for future advancements in material science, environmental protection, medicine, and countless other areas.
Latest Posts
Latest Posts
-
What Is The Opposite Of 9
Mar 31, 2025
-
How Many Neutrons Does Carbon 13 Have
Mar 31, 2025
-
How Do You Find The Mass Of A Cube
Mar 31, 2025
-
What Color Of Light Has The Highest Energy
Mar 31, 2025
-
Balanced Equation Of Magnesium And Hydrochloric Acid
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about An Atom That Gains An Electron Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
