Similarities Between Ionic And Covalent Compounds
listenit
Mar 24, 2025 · 5 min read
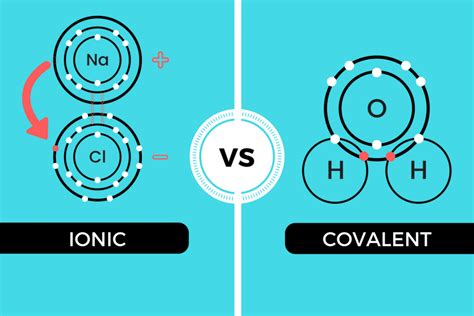
Table of Contents
Unveiling the Similarities Between Ionic and Covalent Compounds: A Deeper Dive
While ionic and covalent compounds are often presented as distinct opposites in chemistry, a closer examination reveals surprising similarities. Understanding these commonalities provides a more nuanced understanding of chemical bonding and the properties of matter. This article will explore the subtle yet significant overlaps between these two fundamental compound types, going beyond the typical textbook distinctions.
Shared Ground: Fundamental Building Blocks
Both ionic and covalent compounds are formed through the interaction of atoms, the fundamental building blocks of matter. They both aim to achieve stability, typically by fulfilling the octet rule (or duet rule for hydrogen) – attaining a full outer electron shell. This drive for stability is the underlying force behind the formation of both types of compounds. While the mechanism differs significantly, the goal remains consistent.
Electron Involvement: The Unifying Factor
At their core, both ionic and covalent bonding involve the interaction of electrons. In ionic bonding, electrons are transferred from one atom to another, creating charged ions. In covalent bonding, electrons are shared between atoms. Although the mode of interaction varies, electron behavior is central to both processes. This shared reliance on electron manipulation creates a fundamental link between these seemingly disparate bonding types.
Formation of Stable Structures: The Ultimate Goal
Both ionic and covalent compounds form stable structures characterized by lower overall energy compared to their constituent atoms. This decrease in energy is a direct consequence of the bonding process, representing the system's drive toward a more stable configuration. The specific nature of the structure – crystalline lattice for ionic compounds, molecules for covalent compounds – differs, but the fundamental principle of energy minimization unites them.
Beyond the Basics: Exploring Deeper Similarities
While the basic mechanisms differ, several less obvious similarities exist between ionic and covalent compounds, challenging the traditional binary classification.
Polarity and Intermolecular Forces: A Shared Spectrum
Contrary to popular belief, not all covalent compounds are nonpolar. Many covalent compounds exhibit polarity due to differences in electronegativity between the bonded atoms. This polarity leads to dipole-dipole interactions, a type of intermolecular force. Interestingly, ionic compounds, while primarily defined by electrostatic interactions between ions, can also experience dipole-dipole interactions if the constituent ions possess a significant charge separation. This shared susceptibility to dipole-dipole forces blurs the lines between the two categories.
Solubility and Conductivity: Exceptions to the Rules
Textbook descriptions often portray ionic compounds as highly soluble in polar solvents and good conductors of electricity when dissolved or molten. Covalent compounds, on the other hand, are typically described as less soluble in polar solvents and poor conductors. However, numerous exceptions exist. Some covalent compounds, like glucose, are highly soluble in water due to their ability to form hydrogen bonds. Similarly, certain ionic compounds have low solubility due to strong lattice energies. Conductivity too is a matter of degree; some covalent compounds can exhibit weak conductivity under specific conditions. These exceptions highlight the limitations of rigid categorization.
Reactivity and Chemical Transformations: A Shared Stage
Both ionic and covalent compounds undergo a wide range of chemical reactions. They can participate in acid-base reactions, redox reactions, and many other types of transformations. The specific reactivity is dictated by the nature of the bonds and the constituent atoms, but the principle of chemical transformation applies equally to both. The products of these reactions might be either ionic or covalent, emphasizing the interconnectivity between these bonding types.
The Role of Electronegativity: A Common Thread
Electronegativity, the ability of an atom to attract electrons in a chemical bond, plays a crucial role in both ionic and covalent bonding. In ionic bonds, a large electronegativity difference leads to complete electron transfer. In covalent bonds, a smaller electronegativity difference results in electron sharing. However, even in covalent bonds, the electronegativity difference affects the bond polarity, impacting the compound's properties. This shared dependence on electronegativity underscores a unifying principle in chemical bonding.
Bridging the Gap: Examples of Overlapping Properties
Let's consider some specific examples to illustrate the overlapping characteristics of ionic and covalent compounds.
Hydrogen Bonding: A Force Transcending Categories
Hydrogen bonding, a strong type of dipole-dipole interaction, occurs in both ionic and covalent compounds. In water (a covalent compound), hydrogen bonds are responsible for its high boiling point and other unique properties. In certain hydrated ionic compounds, hydrogen bonds form between water molecules and the ions, significantly influencing solubility and crystal structure. This illustrates the phenomenon of hydrogen bonding transcends the strict dichotomy of ionic versus covalent.
Amphoteric Compounds: Exhibiting Dual Nature
Amphoteric compounds can act as both acids and bases. Some amphoteric compounds are covalent (e.g., water), while others have significant ionic character. This behavior demonstrates the ability of compounds to display properties associated with both ionic and covalent bonding.
Coordination Compounds: A Blend of Bonding Types
Coordination compounds involve the interaction of a central metal ion (typically cationic) with ligands (often neutral molecules or anions). The bonding within the coordination complex involves both ionic and covalent contributions, highlighting the blending of these bonding types in a single chemical species. This blurring of categories emphasizes the complexity of chemical bonding beyond simple binary classifications.
Conclusion: A Spectrum, Not a Dichotomy
The distinctions between ionic and covalent compounds are essential for understanding fundamental chemical principles. However, a rigid categorization fails to capture the rich complexity of chemical bonding and the properties of matter. Many compounds exhibit characteristics that fall along a spectrum between purely ionic and purely covalent. Recognizing and understanding these similarities allows for a more comprehensive and nuanced view of chemical bonding, moving beyond simplistic dichotomies and embracing the continuous nature of chemical interactions. This deeper understanding enhances our ability to predict and explain the behavior of a vast array of chemical substances. The commonalities highlighted here illuminate the fundamental interconnectedness of different chemical concepts and reinforce the idea that chemical bonding is a continuous spectrum rather than a rigid classification system. By appreciating these subtle overlaps, we gain a more complete and insightful view of the chemical world.
Latest Posts
Latest Posts
-
What Is The Percentage Of 40 Out Of 50
Mar 25, 2025
-
Beryllium On The Periodic Table Of Elements
Mar 25, 2025
-
9 Is 45 Of What Number
Mar 25, 2025
-
How Many Inches Is 1 8 Of A Yard
Mar 25, 2025
-
3 5 6 As An Improper Fraction
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Similarities Between Ionic And Covalent Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
