Real Life Examples Of Gay Lussac's Law
listenit
Mar 22, 2025 · 7 min read
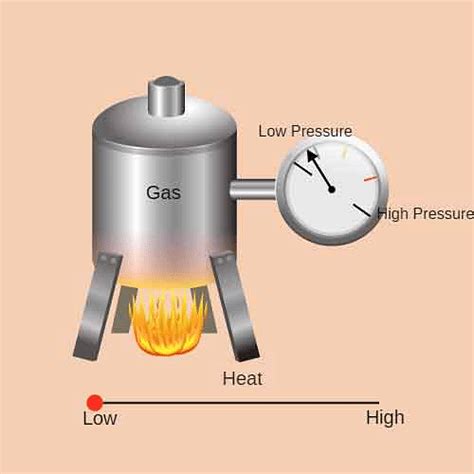
Table of Contents
Real-Life Examples of Gay-Lussac's Law: From Balloons to Pressure Cookers
Gay-Lussac's Law, also known as Amontons' Law, describes the relationship between the pressure and temperature of a gas, holding the volume constant. It states that the pressure of a given amount of gas held at constant volume is directly proportional to the absolute temperature. In simpler terms, as the temperature of a gas increases, its pressure increases proportionally, and vice versa, provided the volume remains unchanged. This seemingly simple law has profound implications and manifests in countless real-life scenarios. Let's delve into some compelling examples:
Everyday Applications of Gay-Lussac's Law
Many everyday occurrences demonstrate the principles of Gay-Lussac's Law, often without us even realizing it. Understanding these examples allows us to appreciate the ubiquitous nature of this fundamental gas law.
1. Pressure Cookers: A Kitchen Application
Pressure cookers are a fantastic example of Gay-Lussac's Law in action. These kitchen appliances work by trapping steam inside a sealed container. As the food cooks and produces steam, the temperature inside the cooker rises. Because the volume is constant (the cooker is sealed), the pressure increases proportionally, according to Gay-Lussac's Law. This increased pressure leads to higher cooking temperatures, resulting in faster cooking times and more tender food. The pressure release valve is crucial for safety; it prevents the pressure from exceeding safe limits and causing potential harm. Understanding the relationship between temperature and pressure in a pressure cooker is essential for safe and effective cooking.
2. Aerosol Cans: Utilizing Pressure Changes
Aerosol cans are another common example. These cans contain a pressurized gas that propels the liquid contents out when the valve is depressed. The gas inside the can is typically compressed and held at a high pressure. Changes in temperature affect this pressure significantly. On a hot day, the temperature of the gas inside increases, leading to a corresponding increase in pressure. This can potentially increase the risk of the can exploding. Conversely, in cold temperatures, the pressure inside the can decreases. This is why it's advisable to store aerosol cans in a cool place and avoid exposing them to extreme temperatures.
3. Hot Air Balloons: A Spectacular Demonstration
The operation of hot air balloons offers a spectacular visual demonstration of Gay-Lussac's Law. Hot air balloons inflate by heating the air inside a large envelope. The heated air expands, but the volume of the envelope remains relatively constant (ignoring minor expansion). This leads to an increase in pressure inside the envelope. However, because the heated air is less dense than the surrounding cooler air, the increased pressure is not a significant safety concern. Instead, the key factor is the difference in density, causing the hot air balloon to rise. The balloon rises because the buoyant force (upward force) exerted by the cooler, denser surrounding air exceeds the weight of the balloon and its contents. As the pilot cools the air within the balloon, the pressure and the buoyant force decrease, allowing the balloon to descend.
4. Tires and Temperature: Maintaining Proper Pressure
The pressure inside car tires or bicycle tires is also influenced by Gay-Lussac's Law. On a hot summer day, the temperature of the air inside the tires increases, resulting in a higher pressure. This can lead to overinflation, potentially causing tire damage or blowouts. Conversely, in cold weather, the air inside the tires cools, causing the pressure to decrease. Underinflation can lead to reduced fuel efficiency and compromised handling. Therefore, regular tire pressure checks, particularly across varying temperatures, are essential for safe driving.
5. Weather Balloons: Reaching High Altitudes
Weather balloons, used for meteorological observations, provide another real-world example. As these balloons ascend into the upper atmosphere, the temperature of the surrounding air decreases significantly. The gas inside the balloon also cools, leading to a decrease in pressure. The balloon expands to maintain a nearly constant internal pressure. If the balloon were rigid, the pressure decrease would be substantial. This illustrates the interplay between temperature, pressure, and volume in a dynamic environment. The balloon's design and materials are crucial to withstand the varying pressures and temperatures experienced at different altitudes.
Industrial and Scientific Applications of Gay-Lussac's Law
Beyond everyday applications, Gay-Lussac's Law plays a critical role in several industrial and scientific processes. Its understanding is fundamental to many engineering disciplines.
6. Internal Combustion Engines: Powering Vehicles
Internal combustion engines rely on the principle of controlled explosions. The combustion of fuel-air mixtures within the engine cylinders generates significant heat, increasing the temperature and pressure of the gases. This increase in pressure, as dictated by Gay-Lussac's Law, drives the pistons, producing mechanical work. Careful control of temperature and pressure is crucial for engine efficiency and longevity. Engineers must carefully design engine components to withstand the high pressures and temperatures generated during combustion.
7. Refrigerators and Air Conditioners: Maintaining Cool Temperatures
Refrigerators and air conditioners use refrigerants that undergo phase changes to cool their surroundings. The process involves compressing the refrigerant gas, increasing its temperature and pressure. This high-pressure, high-temperature gas then releases heat to the environment. The refrigerant then expands, leading to a decrease in temperature and pressure, effectively cooling the interior of the refrigerator or air conditioner. This cycle continually operates, drawing heat from the inside and releasing it to the outside, all in accordance with Gay-Lussac's Law.
8. Gas Pipelines: Managing Pressure in Long-Distance Transport
The transport of natural gas through long-distance pipelines requires careful pressure management. Temperature fluctuations along the pipeline can affect the gas pressure. Understanding Gay-Lussac's Law is crucial for designing and operating these pipelines safely and efficiently. Pressure control systems are essential to ensure that the pressure remains within safe operating limits, preventing potential pipeline damage or leaks.
9. Scuba Diving: Understanding Pressure at Depth
Scuba diving involves changes in pressure with depth. As a diver descends, the pressure of the surrounding water increases. The air in the diver's lungs also experiences this increased pressure. This is not a direct consequence of Gay-Lussac's Law (as volume changes), but rather a consequence of the hydrostatic pressure. However, the air's temperature in the scuba tank does affect the pressure. If the temperature inside the scuba tank changes due to external factors, the internal pressure changes according to Gay-Lussac's Law, which divers must factor into their calculations and safety measures.
10. Rocket Propulsion: Generating Thrust
Rocket propulsion utilizes the expansion of hot gases to generate thrust. The combustion of rocket propellant produces high-temperature, high-pressure gases. These gases are then expelled through a nozzle, producing thrust that propels the rocket forward. The relationship between temperature, pressure, and the expansion of the gases is governed by Gay-Lussac's Law and other gas laws. Careful design and control of the combustion process are crucial for optimizing the performance and efficiency of rocket engines.
Limitations and Considerations
While Gay-Lussac's Law provides a valuable understanding of the relationship between temperature and pressure in gases, it's important to acknowledge its limitations.
-
Ideal Gas Assumption: Gay-Lussac's Law is based on the ideal gas law, which assumes that gas molecules have negligible volume and do not interact with each other. Real gases deviate from this ideal behavior, especially at high pressures and low temperatures.
-
Constant Volume: The law strictly applies only when the volume is held constant. In many real-world situations, volume changes can occur, requiring a more comprehensive approach using the combined gas law or other thermodynamic principles.
-
Specific Gas Properties: The proportionality constant in Gay-Lussac's Law can vary slightly depending on the specific gas being considered.
Conclusion
Gay-Lussac's Law, though simple in its statement, has far-reaching implications in a wide array of applications. From the everyday use of pressure cookers and aerosol cans to sophisticated industrial processes like rocket propulsion and gas pipeline management, understanding the relationship between temperature and pressure is crucial for safety, efficiency, and innovation. While the law's limitations must be acknowledged, its underlying principle remains a cornerstone of our understanding of gases and their behavior in various real-world contexts. Its relevance continues to grow as technology advances and new applications emerge. Appreciating the power of Gay-Lussac's Law allows us to better understand and interact with the world around us.
Latest Posts
Latest Posts
-
Lewis Structure Of Co With Formal Charges
Mar 23, 2025
-
How Many Fluid Ounces Are In 2 Pints
Mar 23, 2025
-
Is 3 5 A Rational Number
Mar 23, 2025
-
What Is The Gcf Of 54 And 72
Mar 23, 2025
-
Is Fungi A Eukaryote Or Prokaryote
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Real Life Examples Of Gay Lussac's Law . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
