Lewis Structure Of Co With Formal Charges
listenit
Mar 23, 2025 · 5 min read
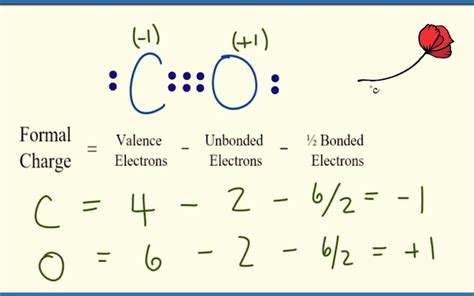
Table of Contents
Lewis Structure of CO with Formal Charges: A Comprehensive Guide
The carbon monoxide molecule (CO) presents a fascinating case study in chemical bonding, challenging our initial intuitions based on simple valence rules. Understanding its Lewis structure, including the distribution of formal charges, is crucial for grasping its unique properties and reactivity. This comprehensive guide delves deep into the intricacies of CO's Lewis structure, exploring multiple resonance structures, formal charge calculations, and the implications for the molecule's behavior.
Understanding Lewis Structures and Formal Charges
Before diving into the specifics of CO, let's establish a foundational understanding of Lewis structures and formal charges. A Lewis structure, also known as an electron dot structure, is a visual representation of the valence electrons in a molecule. It uses dots to represent valence electrons and lines to represent covalent bonds (shared electron pairs). Lewis structures help us predict molecular geometry and reactivity.
Formal charge, on the other hand, is a bookkeeping tool that helps us assess the distribution of electrons in a molecule. It’s calculated for each atom using the following formula:
Formal Charge = (Valence electrons) - (Non-bonding electrons) - (1/2 * Bonding electrons)
A lower formal charge on each atom generally indicates a more stable structure. The ideal scenario is for all atoms to have a formal charge of zero. However, this isn't always achievable, and some molecules exhibit resonance structures with varying formal charges.
Drawing the Lewis Structure of CO: A Step-by-Step Approach
Carbon (C) has four valence electrons, while oxygen (O) has six. Following the steps to draw the Lewis structure:
-
Count Total Valence Electrons: The total number of valence electrons in CO is 4 + 6 = 10.
-
Identify the Central Atom: Carbon is less electronegative than oxygen, so it's usually placed in the center.
-
Form Single Bonds: Connect the carbon and oxygen atoms with a single bond, using two electrons. This leaves 8 electrons remaining.
-
Complete Octet: Assign the remaining eight electrons as lone pairs around the oxygen atom. This completes the oxygen octet, but leaves carbon with only two electrons.
-
Multiple Bonds and Octet Rule Exceptions: To satisfy the octet rule for carbon, we need to form a triple bond between carbon and oxygen. This involves moving two pairs of electrons from oxygen's lone pairs to form two additional bonds with carbon.
The resulting Lewis structure shows a triple bond (≡) between the carbon and oxygen atoms, with two lone pairs remaining on oxygen. This representation, while fulfilling the octet rule, is not the complete picture.
Resonance Structures of CO
Carbon monoxide is a fascinating case in which resonance structures come into play. Resonance structures are multiple possible Lewis structures that can be drawn for a single molecule, each representing a different distribution of electron density. While they aren't different molecules, they contribute to the overall structure and properties.
For CO, we can consider two resonance structures:
Resonance Structure 1: Shows a triple bond between carbon and oxygen, with two lone pairs on oxygen and no formal charges.
Resonance Structure 2: Shows a quadruple bond (though highly unusual) with a formal positive charge on carbon and a formal negative charge on oxygen. This structure is less favored due to the high formal charges, but contributes to the overall resonance hybrid.
The true structure of CO is a resonance hybrid of these structures. The actual bonding is somewhere between a triple bond and a quadruple bond, making the molecule exceptionally stable.
Calculating Formal Charges in CO Resonance Structures
Let's calculate the formal charges for each atom in both resonance structures:
Resonance Structure 1 (Triple Bond):
- Carbon: Formal Charge = 4 - 0 - (1/2 * 6) = +1
- Oxygen: Formal Charge = 6 - 4 - (1/2 * 4) = 0
Resonance Structure 2 (Quadruple Bond): (This structure is significantly less contributing and is primarily discussed for the completeness of understanding resonance)
- Carbon: Formal Charge = 4 - 0 - (1/2 * 8) = 0
- Oxygen: Formal Charge = 6 - 0 - (1/2 * 8) = -2
Implications of Formal Charges and Resonance
The formal charges in the most significant resonance structure (the triple bond structure) highlight an interesting point. While oxygen is more electronegative, the formal charge distribution suggests a partially positive carbon and a neutral oxygen. This is because the triple bond results in a significant electron density shift towards the oxygen atom. This polar nature is why CO has a dipole moment, though it's smaller than might be initially expected given the electronegativity difference.
The resonance hybridization explains the extraordinary stability of the CO molecule. The bonding order, which averages approximately 3, reflects the strength of the bond. It's one of the strongest bonds known in chemistry.
Beyond the Basics: Advanced Concepts
The Lewis structure provides a foundational understanding of CO, but more sophisticated models are needed for a complete picture. These include:
-
Molecular Orbital Theory: This theory offers a more accurate description of bonding, especially in molecules with multiple bonds. It explains the energy levels of the molecular orbitals and the electron distribution within them.
-
Bond Order: The bond order, approximately 3 in CO, relates to the bond strength and length. A higher bond order correlates with a stronger and shorter bond.
-
Spectroscopic Techniques: Techniques like infrared (IR) and Raman spectroscopy provide experimental evidence to support the theoretical models. They allow us to examine the vibrational modes of the molecule and corroborate the presence of a strong triple bond.
Conclusion: Understanding CO's Unique Bonding
The Lewis structure of carbon monoxide, while seemingly simple at first glance, reveals a complex interplay between formal charges, resonance structures, and bonding strength. The molecule’s unique stability and reactivity are direct consequences of this intricate electronic structure. By understanding the formal charges and resonance in CO, we gain a deeper appreciation for the subtleties of chemical bonding and the limitations and successes of Lewis structures in depicting molecular structure and behavior. Further exploration through molecular orbital theory and experimental data provides a more complete and nuanced understanding of this fascinating molecule. This in-depth analysis highlights the importance of considering different theoretical models to fully grasp the complexities of chemical systems.
Latest Posts
Latest Posts
-
How Many Protons Neutrons And Electrons Does Sodium Have
Mar 25, 2025
-
How Many Valence Electrons Are In Ca
Mar 25, 2025
-
One Light Year Is Equal To Km
Mar 25, 2025
-
How Much Is 1 4 Of A Gallon
Mar 25, 2025
-
A Quadrilateral With Four Congruent Sides
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Lewis Structure Of Co With Formal Charges . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
