How Many Protons Neutrons And Electrons Does Sodium Have
listenit
Mar 25, 2025 · 5 min read
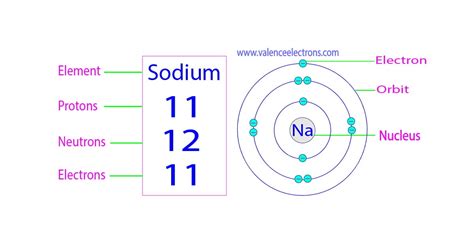
Table of Contents
How Many Protons, Neutrons, and Electrons Does Sodium Have? A Deep Dive into Atomic Structure
Sodium, a ubiquitous element vital for life, holds a fascinating place in the periodic table. Understanding its atomic structure – specifically the number of protons, neutrons, and electrons – is key to grasping its chemical properties and biological importance. This article will delve into the specifics of sodium's atomic composition, explore the concepts of atomic number, mass number, and isotopes, and discuss how this knowledge helps us understand sodium's behavior in various contexts.
Understanding Atomic Structure: The Building Blocks of Matter
Before diving into sodium's specifics, let's establish a foundational understanding of atomic structure. Atoms are the fundamental units of matter, composed of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's identity; it's the atomic number.
- Neutrons: Neutrally charged particles also located in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. They determine the atom's chemical reactivity.
The number of protons and electrons in a neutral atom are always equal, maintaining electrical neutrality. The number of neutrons, however, can vary, leading to the existence of isotopes.
Sodium's Atomic Number and Mass Number
Sodium's atomic number is 11. This means every sodium atom possesses 11 protons in its nucleus. Since a neutral sodium atom has an equal number of protons and electrons, it also contains 11 electrons.
The mass number of an atom represents the total number of protons and neutrons in its nucleus. While the atomic number is constant for a given element, the mass number can vary due to differing numbers of neutrons. The standard atomic mass of sodium is approximately 22.99 atomic mass units (amu). This is a weighted average reflecting the abundance of different sodium isotopes.
Isotopes of Sodium: Variations in Neutron Count
Isotopes are atoms of the same element (same number of protons) but with differing numbers of neutrons. This means they have the same atomic number but different mass numbers. Sodium has several known isotopes, the most stable and abundant being Sodium-23 (²³Na).
-
Sodium-23 (²³Na): This isotope accounts for the vast majority of naturally occurring sodium. It has 11 protons and 12 neutrons (11 + 12 = 23). This is the isotope we typically consider when discussing sodium's properties.
-
Other Sodium Isotopes: Other isotopes of sodium exist, but they are radioactive and have much shorter half-lives. These isotopes are used in various applications like medical imaging and research, but their presence in everyday life is negligible.
Electron Configuration and Chemical Reactivity
The arrangement of electrons in sodium's electron shells significantly influences its chemical behavior. Sodium has 11 electrons, which are distributed across three energy levels:
- First energy level (n=1): 2 electrons
- Second energy level (n=2): 8 electrons
- Third energy level (n=3): 1 electron
This single electron in the outermost shell (valence electron) is readily lost to achieve a stable electron configuration, resembling that of the noble gas neon. This tendency to lose an electron makes sodium highly reactive and readily forms a +1 cation (Na⁺). This explains sodium's prevalence in ionic compounds.
Sodium's Importance in Biology and Chemistry
The unique atomic structure of sodium dictates its crucial roles in biological and chemical systems:
Biological Importance:
- Electrolyte Balance: Sodium is a vital electrolyte, essential for maintaining fluid balance, nerve impulse transmission, and muscle contraction. Its role in maintaining osmotic pressure is paramount for cellular function.
- Nutrient: Sodium is a necessary nutrient in moderate amounts, contributing to various bodily processes.
- Sodium-Potassium Pump: The sodium-potassium pump, a crucial protein in cell membranes, utilizes sodium and potassium ions to regulate cell volume and maintain electrochemical gradients essential for various cellular processes.
Chemical Importance:
- Ionic Compounds: Sodium's tendency to lose an electron and form Na⁺ ions makes it a common component in many ionic compounds, like sodium chloride (table salt, NaCl) and sodium hydroxide (NaOH).
- Sodium Lamps: Sodium vapor lamps utilize sodium's spectral emission to produce a characteristic yellow light, commonly used in street lighting.
- Industrial Applications: Sodium is used in various industrial processes, including the production of chemicals, alloys, and other materials.
Determining the Number of Protons, Neutrons, and Electrons
To summarize the key takeaway:
- Protons: Always 11 (This is defined by the atomic number).
- Electrons: Usually 11 in a neutral atom. This can change if the atom becomes an ion (e.g., Na⁺ has 10 electrons).
- Neutrons: This varies depending on the isotope. The most common isotope, ²³Na, has 12 neutrons.
It's crucial to remember that while the number of protons is constant for a given element, the number of neutrons can differ, resulting in isotopes. This variation doesn't alter the element's fundamental chemical properties but can impact its physical properties, particularly its mass and radioactivity.
Advanced Concepts and Further Exploration
For those interested in a deeper dive, further exploration into the following concepts will provide a more comprehensive understanding of sodium's atomic structure and behavior:
- Nuclear Binding Energy: The energy required to disassemble an atom's nucleus into its constituent protons and neutrons.
- Nuclear Stability: The factors that influence the stability of different isotopes, including the neutron-to-proton ratio.
- Radioactive Decay: The process by which unstable isotopes transform into more stable forms, often involving the emission of particles or energy.
- Mass Spectrometry: A technique used to determine the isotopic composition of a sample, providing insights into the relative abundance of different isotopes.
- Quantum Mechanics: The underlying theoretical framework that describes the behavior of electrons within atoms.
Understanding the fundamental atomic structure of elements like sodium is paramount for comprehending their chemical and physical properties, and their significant roles in various scientific disciplines, from biology and chemistry to materials science and engineering. This knowledge forms the cornerstone of our understanding of the world around us.
Latest Posts
Latest Posts
-
What Is 3 10 In A Decimal
Mar 28, 2025
-
Whats The Sum Of 2 5 And 2 4
Mar 28, 2025
-
Lewis Dot Structure For Magnesium Chloride
Mar 28, 2025
-
Is Na A Solid Liquid Or Gas
Mar 28, 2025
-
How Does Mrna Exit The Nucleus
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Neutrons And Electrons Does Sodium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
