Protons Neutrons And Electrons Of Bromine
listenit
Mar 30, 2025 · 6 min read
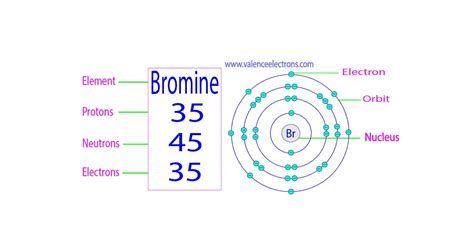
Table of Contents
Protons, Neutrons, and Electrons of Bromine: A Deep Dive into Atomic Structure
Bromine, a fascinating element with a rich history and unique properties, provides an excellent case study for understanding the fundamental building blocks of matter: protons, neutrons, and electrons. This article will delve deep into the atomic structure of bromine, exploring its subatomic particles, their arrangement, and the implications for bromine's chemical behavior and physical properties. We will also touch upon the isotopic variations of bromine and how these affect its overall characteristics.
Understanding the Basics: Subatomic Particles
Before we specifically examine bromine, let's refresh our understanding of the three fundamental subatomic particles:
Protons: The Positive Charge Carriers
Protons are positively charged particles residing within the atom's nucleus. Their mass is approximately 1 atomic mass unit (amu), and their positive charge is equal in magnitude but opposite in sign to the electron's negative charge. The number of protons in an atom's nucleus defines its atomic number and uniquely identifies the element. This is crucial: All bromine atoms, regardless of their isotopic variation, have the same number of protons.
Neutrons: The Neutral Partners
Neutrons, as their name suggests, carry no electrical charge. They are also located within the atom's nucleus and have a mass slightly larger than that of a proton (approximately 1 amu). Neutrons play a vital role in stabilizing the nucleus, particularly in heavier atoms. The number of neutrons in an atom can vary, leading to isotopes of the same element.
Electrons: The Negative Orbitals
Electrons are negatively charged particles with a mass significantly smaller than that of protons or neutrons (approximately 1/1836 amu). They occupy regions of space surrounding the nucleus called electron shells or orbitals. These orbitals are characterized by different energy levels, and electrons fill these shells according to specific rules governed by quantum mechanics. The number of electrons in a neutral atom is equal to the number of protons.
Bromine's Atomic Structure: A Closer Look
Bromine (Br), with its atomic number 35, has 35 protons in its nucleus. This defining characteristic sets it apart from all other elements. Because it's a neutral atom, it also possesses 35 electrons arranged in various energy levels surrounding the nucleus. The arrangement of these electrons dictates bromine's chemical reactivity and its ability to form bonds with other atoms.
The electronic configuration of bromine is [Ar] 3d<sup>10</sup> 4s<sup>2</sup> 4p<sup>5</sup>. This configuration indicates that the electrons are distributed across different energy levels and subshells. The outermost shell (valence shell) contains 7 electrons. This incomplete valence shell is responsible for bromine's high reactivity and tendency to form chemical bonds. It readily gains one electron to achieve a stable octet configuration, making it a highly electronegative element.
Bromine's Neutrons: Isotopes and Stability
Unlike the fixed number of protons and electrons, the number of neutrons in bromine atoms can vary. This variation leads to the existence of bromine isotopes. The most common isotopes are bromine-79 (<sup>79</sup>Br) and bromine-81 (<sup>81</sup>Br).
- Bromine-79 (<sup>79</sup>Br): This isotope has 35 protons and 44 neutrons. It constitutes approximately 50.7% of naturally occurring bromine.
- Bromine-81 (<sup>81</sup>Br): This isotope has 35 protons and 46 neutrons. It makes up approximately 49.3% of naturally occurring bromine.
The presence of two stable isotopes with nearly equal abundance is a unique feature of bromine. The differing numbers of neutrons influence the overall atomic mass, slightly altering some physical properties. However, their chemical properties remain essentially identical, as the number of protons and electrons, determining chemical behavior, is unchanged.
Implications of Bromine's Atomic Structure
The specific arrangement of protons, neutrons, and electrons in bromine has significant implications for its properties and behavior:
Chemical Reactivity: The Drive for Stability
Bromine's 7 valence electrons drive its chemical reactivity. It readily forms covalent bonds with other atoms, particularly those that can provide the single electron needed to complete its valence shell. This explains why bromine is a halogen and shares chemical similarities with other halogens such as chlorine and iodine. Bromine often exists as a diatomic molecule (Br<sub>2</sub>), sharing an electron pair with another bromine atom to achieve a stable octet configuration.
Physical Properties: A Liquid Halogen
The relatively strong intermolecular forces between bromine molecules, arising from the electron distribution and polarization, contribute to its physical properties. At room temperature, bromine exists as a dark reddish-brown liquid – a unique characteristic amongst the halogens. Its relatively high density and boiling point are also a consequence of these intermolecular interactions.
Isotopic Effects: Subtle Variations
While the chemical properties of bromine are largely determined by its proton and electron configuration, the isotopic variations can lead to minor differences in some physical properties. For example, the slightly different masses of <sup>79</sup>Br and <sup>81</sup>Br can lead to measurable differences in diffusion rates and spectroscopic characteristics. However, these variations are relatively small and don't drastically alter bromine's overall behavior.
Bromine's Applications: A Versatile Element
Understanding bromine's atomic structure is crucial to appreciating its wide-ranging applications. Its reactive nature makes it essential in various industrial processes:
-
Flame Retardants: Brominated compounds are used extensively as flame retardants in various materials, from textiles to electronics. The bromine atoms disrupt the combustion process, hindering the spread of fire.
-
Agricultural Chemicals: Certain bromine-containing compounds function as pesticides and fumigants, effectively controlling pests and diseases in agriculture.
-
Pharmaceuticals: Bromine is incorporated into some pharmaceutical products, playing roles in the synthesis of medicines and their effectiveness.
-
Water Treatment: Bromine compounds are used as disinfectants in water treatment processes, effectively eliminating harmful bacteria and pathogens.
-
Dye Synthesis: Certain bromine compounds are used in the production of dyes and pigments, contributing to the vibrant colors found in various materials.
Conclusion: The Significance of Subatomic Structure
The exploration of bromine's protons, neutrons, and electrons offers a fascinating glimpse into the fundamental principles of atomic structure. The number of protons defines bromine's identity, while the number of neutrons influences its isotopic variations. The arrangement of electrons determines its chemical reactivity and how it interacts with other elements, leading to its wide range of applications. Understanding the interplay between these subatomic particles is crucial not only for comprehending bromine's properties but also for appreciating the fundamental building blocks of all matter. Further research and exploration continue to unveil more about the intricate world of atoms and their constituents, constantly expanding our knowledge and capabilities. The continuing investigation into the behavior of subatomic particles promises to drive further innovation and application in numerous fields.
Latest Posts
Latest Posts
-
What Is Square Root Of 63
Apr 01, 2025
-
Least Common Multiple 2 And 4
Apr 01, 2025
-
How Does Igneous Rock Become Metamorphic
Apr 01, 2025
-
How Does Friction Affect The Motion Of Objects
Apr 01, 2025
-
Sr Oh 2 Strong Or Weak
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Protons Neutrons And Electrons Of Bromine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
