Protons Neutrons And Electrons In Sodium
listenit
Mar 24, 2025 · 5 min read
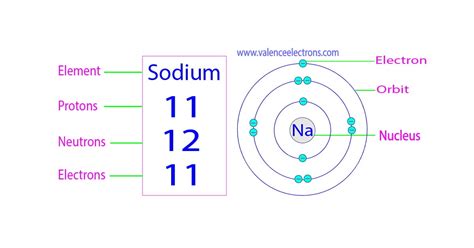
Table of Contents
Protons, Neutrons, and Electrons in Sodium: A Deep Dive into Atomic Structure
Sodium (Na), a highly reactive alkali metal, plays a crucial role in various biological and industrial processes. Understanding its atomic structure, specifically the number and arrangement of its protons, neutrons, and electrons, is key to comprehending its properties and behavior. This article delves into the specifics of sodium's subatomic composition, exploring its implications for chemical bonding, reactivity, and overall characteristics.
Sodium's Atomic Number and Mass Number: Unveiling the Core Components
The atomic number of an element defines its identity and is determined by the number of protons residing within its nucleus. Sodium's atomic number is 11, signifying the presence of 11 protons in its nucleus. These positively charged particles contribute significantly to the atom's overall mass and determine its place on the periodic table.
The mass number of an atom represents the total number of protons and neutrons found in its nucleus. While the number of protons remains constant for a given element, the number of neutrons can vary, leading to isotopes. The most common isotope of sodium is Sodium-23 (²³Na), possessing a mass number of 23. This means it contains 11 protons and 12 neutrons (23 - 11 = 12). These neutrons, while electrically neutral, contribute significantly to the atom's mass and nuclear stability.
Isotopes of Sodium: Exploring Variations in Neutron Count
Isotopes are atoms of the same element that differ in their neutron count. While all isotopes of sodium have 11 protons, their neutron numbers can vary. For instance, while ²³Na is the most prevalent isotope, others exist, including ²²Na, a radioactive isotope with 11 protons and 11 neutrons. These variations in neutron count can affect the stability and properties of the isotope, with radioactive isotopes undergoing decay to achieve greater stability.
Understanding Isotopic Abundance: The abundance of each isotope varies naturally. ²³Na represents the vast majority of naturally occurring sodium, with its stability contributing to its dominance. Radioactive isotopes, like ²²Na, exist in far smaller quantities due to their inherent instability and radioactive decay processes.
Electrons: The Orbital Dance and Chemical Reactivity
The electron cloud surrounding the nucleus contains negatively charged electrons, balancing the positive charge of the protons. In a neutral sodium atom, the number of electrons equals the number of protons – 11 electrons. These electrons are not randomly distributed but occupy specific energy levels or shells, arranged according to quantum mechanical principles.
Electron Configuration: A Structured Arrangement
The electronic configuration of sodium is 1s²2s²2p⁶3s¹. This notation describes the distribution of electrons across different energy levels:
- 1s²: Two electrons occupy the lowest energy level (n=1), the 1s orbital.
- 2s²: Two electrons occupy the 2s orbital in the second energy level (n=2).
- 2p⁶: Six electrons occupy the three 2p orbitals in the second energy level.
- 3s¹: A single electron occupies the 3s orbital in the third energy level (n=3).
This outermost electron in the 3s orbital is crucial for understanding sodium's reactivity. It's relatively loosely bound to the nucleus and readily participates in chemical bonding.
Valence Electrons and Chemical Bonding: The Key to Reactivity
The valence electron is the electron in the outermost shell that participates in chemical reactions. In sodium, this is the single electron in the 3s orbital. Sodium's strong tendency to lose this valence electron to achieve a stable electron configuration (like that of neon, with a filled outer shell) makes it highly reactive. This loss of an electron transforms the sodium atom into a positively charged ion, denoted as Na⁺, also known as a cation.
Sodium's Ionic Bonding: An Example of Electrostatic Attraction
Sodium's reactivity is prominently displayed in its formation of ionic bonds. Ionic bonds are formed through the electrostatic attraction between oppositely charged ions. Sodium readily loses its valence electron to elements such as chlorine (Cl), which readily accepts an electron to complete its outermost electron shell. This results in the formation of sodium chloride (NaCl), commonly known as table salt.
In NaCl, sodium exists as a positively charged ion (Na⁺) and chlorine as a negatively charged ion (Cl⁻). The electrostatic attraction between these oppositely charged ions forms the strong ionic bond holding the crystal lattice of sodium chloride together.
Nuclear Properties and Radioactivity: Exploring Unstable Isotopes
While ²³Na is stable, other isotopes of sodium exhibit radioactivity. Radioactive isotopes undergo nuclear decay, transforming into different isotopes or elements while emitting radiation. The radioactive decay of these isotopes can have both beneficial and harmful effects, depending on the type of radiation emitted and the level of exposure.
Understanding Radioactive Decay Modes: Sodium isotopes may undergo various decay modes, including beta decay, where a neutron is converted into a proton, emitting an electron (beta particle) and an antineutrino. The specifics of the decay process depend on the specific isotope and its nuclear stability.
Applications of Sodium and its Isotopes: From Everyday Life to Scientific Research
Sodium and its isotopes find numerous applications across diverse fields:
-
Sodium in Everyday Life: Sodium chloride (NaCl) is essential in our diets, playing a vital role in maintaining electrolyte balance. Sodium compounds are also used in various industrial applications, including manufacturing of soaps, detergents, and other chemicals.
-
Radioactive Sodium in Medicine and Research: Radioactive isotopes of sodium, such as ²²Na, are utilized in medical imaging techniques, such as PET scans (Positron Emission Tomography). They also find applications in scientific research, particularly in studies involving tracer techniques.
Conclusion: A Comprehensive Understanding of Sodium's Atomic Structure
Understanding the composition and arrangement of protons, neutrons, and electrons in sodium is fundamental to grasping its chemical and physical properties. The presence of 11 protons defines its identity, while the 12 neutrons in the most common isotope contribute to its mass and stability. The single valence electron dictates its reactivity, leading to the formation of ionic bonds and its involvement in diverse chemical reactions. Further exploration of its isotopic variations reveals insights into nuclear properties and applications in various fields. This comprehensive understanding of sodium's atomic structure highlights the interconnectedness of subatomic particles and their impact on the macroscopic properties and applications of this essential element.
Latest Posts
Latest Posts
-
Can Two Different Numbers Have The Same Absolute Value
Mar 26, 2025
-
How Many Neutrons Are In C14
Mar 26, 2025
-
Geometric Mean Of 3 And 12
Mar 26, 2025
-
What Is Greater 3 4 Or 2 3
Mar 26, 2025
-
How Can An Igneous Rock Become A Metamorphic Rock
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Protons Neutrons And Electrons In Sodium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
