Number Of Valence Electrons In Silicon
listenit
Apr 01, 2025 · 6 min read
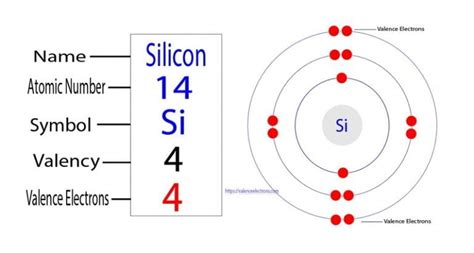
Table of Contents
The Significance of Valence Electrons: Delving Deep into Silicon's Four
Silicon, the heart of the modern digital revolution, owes its remarkable properties to a seemingly simple aspect of its atomic structure: its four valence electrons. Understanding the number and behavior of these electrons is crucial to grasping silicon's role in semiconductors, solar cells, and countless other applications. This article will delve deep into the significance of silicon's four valence electrons, exploring its impact on bonding, conductivity, and its overall contribution to modern technology.
What are Valence Electrons?
Before we focus specifically on silicon, let's establish a foundational understanding of valence electrons. These are the electrons located in the outermost shell, or energy level, of an atom. They are the key players in chemical bonding, determining an atom's reactivity and the types of bonds it can form. The number of valence electrons directly influences an element's position in the periodic table and its chemical properties. Elements with similar numbers of valence electrons often exhibit similar chemical behaviors, a pattern reflected in the periodic table's arrangement.
The Significance of the Outermost Shell
The outermost shell, also known as the valence shell, is the most energetically accessible region for electron interaction. Electrons in this shell are relatively loosely bound to the nucleus and are therefore more likely to participate in chemical reactions. Atoms strive for stability, often achieved by achieving a full valence shell, a configuration that mirrors the stable electron configurations of noble gases. This drive for stability dictates how atoms bond with each other, forming molecules and compounds.
Silicon's Four Valence Electrons: A Foundation for Semiconductors
Silicon, with its atomic number 14, possesses an electronic configuration of 1s²2s²2p⁶3s²3p². This configuration reveals that silicon has four electrons in its outermost shell—the 3s and 3p orbitals. These four valence electrons are the cornerstone of silicon's semiconducting properties, making it an indispensable material in modern electronics.
Covalent Bonding in Silicon
Silicon atoms achieve stability through covalent bonding. Each silicon atom shares its four valence electrons with four neighboring silicon atoms, creating a strong, three-dimensional network of interconnected atoms. This tetrahedral arrangement, where each silicon atom is bonded to four others, is the defining characteristic of silicon's crystalline structure. This robust network is responsible for silicon's high melting point and mechanical strength.
The Role of Doping in Semiconductor Functionality
The beauty of silicon's four valence electrons lies in its ability to be easily manipulated. The process of doping introduces impurity atoms with a different number of valence electrons into the silicon crystal lattice. This deliberate alteration of the electron concentration dramatically affects silicon's electrical conductivity, transforming it from an insulator into a semiconductor.
N-type Doping
Introducing phosphorus (five valence electrons) into a silicon crystal lattice creates n-type silicon. The extra electron from each phosphorus atom becomes a free electron, readily available to conduct electricity. These free electrons are the primary charge carriers in n-type silicon.
P-type Doping
Boron (three valence electrons), when introduced into silicon, produces p-type silicon. Each boron atom creates a "hole" – a missing electron – in the silicon lattice. These holes act as positive charge carriers, allowing current to flow through the material.
The P-N Junction: The Heart of Semiconductor Devices
The magic happens when n-type and p-type silicon are joined to form a p-n junction. At the junction, electrons from the n-type region diffuse into the p-type region, filling the holes. This diffusion creates a depletion region, devoid of charge carriers, and establishes a potential barrier across the junction. This potential barrier can be controlled by applying an external voltage, allowing for the switching and amplification of electrical signals—the fundamental principle behind transistors and diodes.
Silicon's Impact on Technology: A Revolution Driven by Four Electrons
The unique properties arising from silicon's four valence electrons have revolutionized technology. Its role extends far beyond the realm of microelectronics:
Microelectronics: The Silicon Revolution
Silicon's dominance in microelectronics stems directly from its semiconducting properties. The ability to precisely control its conductivity through doping has enabled the creation of incredibly complex integrated circuits (ICs) that power our computers, smartphones, and countless other electronic devices. The miniaturization of transistors, driven by advancements in silicon-based technology, has led to exponentially increasing computing power over the past few decades – a phenomenon known as Moore's Law.
Solar Cells: Harvesting Solar Energy
Silicon's ability to absorb sunlight and convert it into electricity forms the basis of silicon-based solar cells. When sunlight strikes a silicon solar cell, it excites electrons in the silicon lattice, generating an electrical current. The efficiency of these solar cells depends on factors like the purity of the silicon and the design of the cell, but the fundamental principle relies on the interaction of photons with silicon's electrons.
Other Applications
Silicon's versatility extends beyond electronics and solar energy. It finds use in:
- Ceramics and Glass: Silicon dioxide (SiO2), commonly known as silica, is a major component of glass and various ceramics. The strong silicon-oxygen bonds contribute to the durability and heat resistance of these materials.
- Silicones: These polymers, containing silicon-oxygen and silicon-carbon bonds, exhibit unique properties such as water repellency, heat resistance, and flexibility, making them useful in a wide range of applications, from lubricants to medical implants.
- Metallurgy: Silicon is used as an alloying agent in various metals, improving their properties such as strength and castability.
Beyond Silicon: Exploring Other Semiconductors
While silicon reigns supreme in current semiconductor technology, other materials are being explored to address the limitations of silicon at smaller scales and for specialized applications. These materials, such as gallium arsenide (GaAs) and gallium nitride (GaN), possess different bandgaps and electron mobilities, offering advantages in certain applications, such as high-frequency electronics and high-power applications. However, silicon's mature manufacturing processes and cost-effectiveness ensure its continued dominance in the near future.
The Future of Silicon: Challenges and Opportunities
Despite its remarkable success, silicon technology faces challenges. As transistors continue to shrink, quantum effects become increasingly significant, impacting device performance and reliability. Research focuses on overcoming these challenges, exploring new materials and manufacturing techniques to maintain the pace of miniaturization and enhance performance. The exploration of new architectures and materials is crucial for continued innovation in computing and electronics.
Conclusion: Four Electrons, Limitless Possibilities
Silicon's four valence electrons, a seemingly simple feature of its atomic structure, have driven an unprecedented technological revolution. Understanding the behavior of these electrons is crucial to grasping silicon's remarkable properties and its profound impact on our world. From microelectronics to solar energy, silicon's contribution continues to shape our modern lives, and future research promises even greater advancements based on this fundamental element of modern technology. The exploration of silicon's potential continues, pushing the boundaries of what's possible and promising even more innovative applications in the years to come. The seemingly simple presence of four valence electrons unlocks a world of possibilities, a testament to the power of basic science in shaping our technological landscape. The enduring significance of silicon's atomic structure underscores the fundamental role of materials science in driving technological progress.
Latest Posts
Latest Posts
-
An Organism That Eats Only Plants
Apr 02, 2025
-
What Is 1 To The 5th Power
Apr 02, 2025
-
Takes The Place Of A Noun
Apr 02, 2025
-
What Is The Fraction Of 0 04
Apr 02, 2025
-
How Does The Use Of Fertilizer Affect The Nitrogen Cycle
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Silicon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
