Number Of Valence Electrons In Potassium
listenit
Mar 26, 2025 · 6 min read
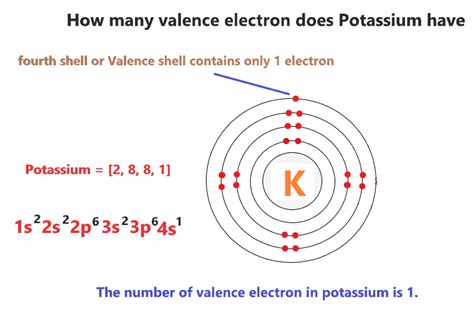
Table of Contents
Unveiling the Secrets of Potassium: A Deep Dive into its Valence Electrons
Potassium, a vital element for life, plays a crucial role in various biological processes. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its chemical behavior and biological significance. This comprehensive article delves into the intricacies of potassium's valence electrons, exploring its atomic structure, chemical reactivity, and biological importance. We'll also explore related concepts and answer frequently asked questions to provide a complete and insightful understanding of this fascinating element.
Understanding Atomic Structure and Valence Electrons
Before we focus on potassium specifically, let's establish a solid foundation in atomic structure and the concept of valence electrons. Atoms are the fundamental building blocks of matter, composed of a nucleus containing protons and neutrons, surrounded by orbiting electrons. These electrons occupy specific energy levels or shells.
The valence shell is the outermost electron shell of an atom. The electrons residing in this shell are called valence electrons. These electrons are crucial because they determine an atom's chemical properties and how it interacts with other atoms to form chemical bonds. The number of valence electrons dictates an atom's reactivity; atoms tend to gain, lose, or share valence electrons to achieve a stable electron configuration, often referred to as the "octet rule" (eight electrons in the valence shell).
Potassium's Electronic Configuration: A Closer Look
Potassium (K), with an atomic number of 19, possesses 19 protons and 19 electrons in a neutral atom. Its electronic configuration can be represented as 1s²2s²2p⁶3s²3p⁶4s¹. This configuration reveals the distribution of electrons across different energy levels.
-
Inner Shells: The inner shells (1s², 2s², 2p⁶, 3s², 3p⁶) are completely filled, meaning they are stable and unreactive.
-
Valence Shell: The outermost shell, the 4s orbital, contains only one electron. This single electron is potassium's valence electron.
Therefore, potassium has only one valence electron.
Potassium's Chemical Reactivity: The Role of the Valence Electron
The presence of just one valence electron profoundly impacts potassium's chemical reactivity. Atoms strive for stability, often achieved by having a full valence shell. Potassium readily loses its single valence electron to achieve the stable electron configuration of the noble gas Argon (Ar), which has a filled 3p subshell. This process results in the formation of a positively charged potassium ion (K⁺).
This ease of losing an electron makes potassium a highly reactive element, particularly with elements that readily accept electrons, such as halogens (e.g., chlorine, bromine, iodine). The reaction between potassium and a halogen produces an ionic compound, where potassium exists as a K⁺ ion and the halogen as a negatively charged ion. The electrostatic attraction between these oppositely charged ions forms the ionic bond.
Examples of Potassium's Reactivity:
-
Reaction with water: Potassium reacts violently with water, producing hydrogen gas and potassium hydroxide. The reaction is highly exothermic (releases a significant amount of heat).
-
Reaction with halogens: Potassium readily reacts with halogens to form ionic salts such as potassium chloride (KCl), potassium bromide (KBr), and potassium iodide (KI).
-
Reaction with oxygen: Potassium reacts with oxygen in the air to form potassium oxide (K₂O).
Potassium's Biological Significance: A Vital Element
Potassium's chemical properties make it essential for numerous biological processes. Its ability to carry a positive charge allows it to play critical roles in:
-
Maintaining electrolyte balance: Potassium is a major electrolyte in the body, essential for maintaining the proper balance of fluids and electrolytes within cells and across cell membranes. This balance is crucial for nerve and muscle function.
-
Nerve impulse transmission: The movement of potassium ions across cell membranes is vital for transmitting nerve impulses. Changes in potassium concentration trigger the depolarization and repolarization of nerve cells, enabling communication throughout the nervous system.
-
Muscle contraction: Similar to nerve impulse transmission, potassium ion movement is crucial for muscle contraction. Proper potassium levels are essential for the coordinated contraction and relaxation of muscles.
-
Maintaining blood pressure: Potassium plays a role in regulating blood pressure. Appropriate potassium levels help maintain healthy blood pressure by influencing the constriction and dilation of blood vessels.
-
Enzyme activity: Potassium ions act as cofactors for many enzymes, assisting in their catalytic activity. This means they are crucial for countless biochemical reactions in the body.
Deficiencies in potassium (hypokalemia) can lead to serious health problems, including muscle weakness, fatigue, heart irregularities, and even paralysis. Conversely, excessive potassium levels (hyperkalemia) can also be dangerous and affect heart function. Therefore, maintaining healthy potassium levels through a balanced diet is crucial for overall health.
Beyond the Basics: Exploring Related Concepts
Understanding potassium's valence electron necessitates exploring several related concepts:
-
Ionization Energy: This refers to the energy required to remove an electron from an atom or ion. Potassium has a relatively low ionization energy, indicating the ease with which it loses its valence electron.
-
Electronegativity: This measures an atom's ability to attract electrons in a chemical bond. Potassium has low electronegativity, meaning it is less likely to attract electrons and more likely to lose its valence electron.
-
Oxidation States: The oxidation state of an atom indicates its apparent charge in a compound. Potassium typically exhibits an oxidation state of +1, reflecting the loss of its single valence electron.
-
Periodic Trends: The number of valence electrons and related properties like ionization energy and electronegativity follow predictable trends within the periodic table. Potassium's position in Group 1 (alkali metals) reflects its characteristic properties.
Frequently Asked Questions (FAQs)
Q: Why is potassium so reactive?
A: Potassium's high reactivity stems from its single valence electron. It readily loses this electron to achieve a stable, filled electron shell, resulting in a stable ion.
Q: What happens when potassium loses its valence electron?
A: When potassium loses its valence electron, it forms a positively charged ion (K⁺), achieving a stable electron configuration similar to Argon.
Q: What are the health consequences of potassium deficiency?
A: Potassium deficiency (hypokalemia) can lead to muscle weakness, fatigue, heart irregularities, constipation, and potentially life-threatening conditions.
Q: How can I ensure I get enough potassium in my diet?
A: Potassium-rich foods include bananas, potatoes, spinach, tomatoes, and beans. Consult a healthcare professional for personalized dietary advice.
Q: How does potassium differ from other alkali metals?
A: While potassium shares similarities with other alkali metals (Li, Na, Rb, Cs, Fr) – all possessing one valence electron – it differs slightly in reactivity and atomic size. Its reactivity is less than caesium and rubidium but higher than sodium and lithium.
Conclusion: The Significance of Potassium's Single Valence Electron
Potassium's single valence electron is the key to understanding its chemical and biological properties. Its reactivity, its ability to form stable ionic compounds, and its crucial role in various biological processes are all directly linked to this single electron. By understanding the intricacies of its electronic structure, we gain valuable insights into its chemical behavior and its essential contributions to life itself. This exploration underscores the importance of studying atomic structure to comprehend the behavior and function of elements in both the chemical and biological realms. Further research into the specific interactions of potassium's valence electron will continue to shed light on its significant role in various scientific fields.
Latest Posts
Latest Posts
-
The Outermost Layer Of The Kidney
Mar 29, 2025
-
X 2 6x 9 0 Graph
Mar 29, 2025
-
What Is The Lcm Of 15 And 6
Mar 29, 2025
-
How Many Feet Are In A Hundred Yards
Mar 29, 2025
-
What Is The Mass In Grams Of 5 90 Mol C8h18
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Potassium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
