Number Of Valence Electrons In Iron
listenit
Mar 29, 2025 · 6 min read
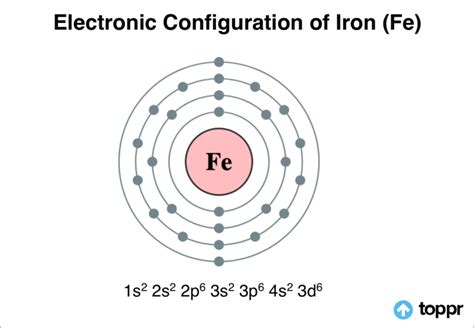
Table of Contents
The Number of Valence Electrons in Iron: A Deep Dive
Iron, a ubiquitous element crucial to life and industry, presents an interesting case study when examining valence electrons. Understanding its valence electron configuration is key to understanding its chemical properties, its reactivity, and its myriad applications. This article will delve deep into the number of valence electrons in iron, exploring its electronic configuration, its implications for bonding, and its significance in various contexts.
Understanding Valence Electrons
Before diving into the specifics of iron, let's establish a firm understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (also known as the valence shell) of an atom. These electrons are the most loosely held and therefore participate in chemical bonding. The number of valence electrons determines an element's reactivity and the types of bonds it can form (ionic, covalent, metallic). The valence shell configuration dictates the element's chemical behavior and its position within the periodic table.
Iron's Electronic Configuration and Valence Electrons
Iron (Fe) has an atomic number of 26, meaning it possesses 26 protons and, in its neutral state, 26 electrons. To determine the number of valence electrons, we must understand its electronic configuration. This configuration describes how electrons are distributed among the various energy levels and sublevels within the atom.
Iron's electronic configuration is 1s²2s²2p⁶3s²3p⁶4s²3d⁶. This can also be represented in a shorthand notation using the previous noble gas Argon (Ar) as a base: [Ar] 4s²3d⁶.
Now, the crucial part: identifying the valence electrons. While the general rule of thumb is to consider only the outermost shell (the highest principal quantum number, 'n'), iron's case requires a nuanced approach due to the involvement of the d-orbital.
In many transition metals, including iron, the 4s electrons and 3d electrons are considered valence electrons because their energy levels are close enough that both can participate in bonding. Therefore, iron has eight valence electrons (two 4s electrons and six 3d electrons).
Why the Ambiguity with Transition Metals?
The straightforward "outermost shell" rule works well for main group elements (s and p block). However, transition metals (d block) introduce complexity. The energy difference between the (n-1)d and ns orbitals is small, meaning both can contribute to chemical bonding.
This proximity in energy levels means that the 3d and 4s orbitals in iron can both participate in chemical bonding, leading to variable oxidation states. This is a defining characteristic of transition metals. Unlike main group elements that usually exhibit a single, predictable oxidation state, transition metals, like iron, often exhibit multiple oxidation states.
Iron's Variable Oxidation States and Valence Electrons
Iron's ability to form multiple oxidation states directly stems from its valence electrons. It commonly exists in the +2 (ferrous) and +3 (ferric) oxidation states.
-
Fe²⁺ (Ferrous): In this state, iron loses two electrons, typically the two 4s electrons. This leaves six 3d electrons remaining.
-
Fe³⁺ (Ferric): In this state, iron loses three electrons. This typically involves losing two 4s electrons and one 3d electron.
This variability in oxidation states is a consequence of the relatively easy removal of electrons from both the 4s and 3d orbitals. This flexibility is a crucial factor in iron's diverse chemical behavior and its ability to form a wide range of compounds.
Implications of Iron's Valence Electrons: Chemical Bonding
The eight valence electrons in iron directly influence the types of bonds it forms:
-
Metallic Bonding: In pure iron, the valence electrons are delocalized, forming a "sea" of electrons that holds the iron atoms together through metallic bonding. This accounts for iron's characteristic properties like high electrical and thermal conductivity, malleability, and ductility.
-
Ionic Bonding: When iron forms ionic compounds, it loses electrons to achieve a stable electron configuration. This electron loss, involving its valence electrons, leads to the formation of ionic bonds with anions like oxygen (O²⁻) or chlorine (Cl⁻). For instance, iron forms ionic compounds like iron(II) oxide (FeO) and iron(III) oxide (Fe₂O₃).
-
Covalent Bonding: While less common than ionic or metallic bonding for iron, it can participate in covalent bonds, especially in complex compounds and organometallic complexes. In these cases, the valence electrons are shared between iron and other atoms.
The Significance of Iron's Valence Electrons in Various Contexts
The implications of iron's valence electron configuration extend far beyond simple chemical bonding. Its properties, directly derived from its electronic structure, make it crucial in various areas:
1. Biological Systems:
Iron's role in biological systems is paramount. Hemoglobin, the protein responsible for oxygen transport in blood, relies on iron's ability to form coordination complexes with oxygen. This ability is a direct result of iron's d-orbital valence electrons and its ability to readily change its oxidation state. Similar iron-containing proteins (cytochromes) play crucial roles in electron transport chains vital for cellular respiration.
2. Metallurgy and Industry:
Iron's metallic properties, a direct consequence of its valence electron delocalization, make it a cornerstone of the metallurgical industry. The ability to easily shape and manipulate iron, combined with its strength and durability, has made it indispensable in construction, manufacturing, and countless other applications. Steel, an alloy of iron and carbon, showcases enhanced properties due to modifications in the electron interactions caused by the carbon atoms.
3. Catalysis:
Iron's ability to readily change its oxidation state, facilitating electron transfer, makes it a vital component in various catalytic processes. Iron-based catalysts play significant roles in industrial chemical reactions, including ammonia production (Haber-Bosch process) and Fischer-Tropsch synthesis of hydrocarbons.
4. Magnetic Properties:
Iron's unique magnetic properties are also directly linked to its electronic configuration. The unpaired electrons in the d-orbital contribute to its ferromagnetic behavior, making it crucial in the creation of magnets and various magnetic storage devices.
Conclusion: The Importance of Understanding Iron's Valence Electrons
The number of valence electrons in iron, while seemingly a simple concept, holds profound implications for its properties and applications. Understanding its eight valence electrons—two from the 4s and six from the 3d orbitals—is key to understanding its variable oxidation states, its diverse bonding capabilities, and its critical role in biological systems, metallurgy, catalysis, and magnetism. This deep understanding allows scientists and engineers to harness iron's unique properties to create materials and technologies with far-reaching impact on our lives. Further research continues to expand our understanding of iron’s behavior in diverse chemical environments, always stemming from the fundamental nature of its valence electrons. This understanding is a cornerstone of modern chemistry and materials science.
Latest Posts
Latest Posts
-
What Is The Gcf Of 27 And 45
Apr 01, 2025
-
Compounds Containing Only Carbon And Hydrogen Are Called
Apr 01, 2025
-
What Is The Percent Of 5 12
Apr 01, 2025
-
How Many Quarts Is 1 L
Apr 01, 2025
-
Sketch The Region Enclosed By The Given Curves
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Iron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
