Number Of Valence Electrons For Sodium
listenit
Mar 24, 2025 · 6 min read
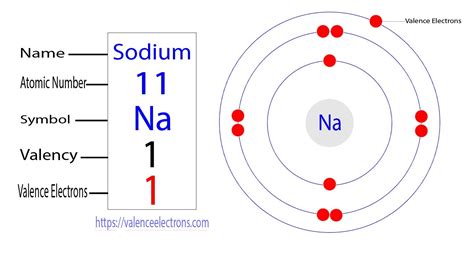
Table of Contents
Unveiling the Secrets of Sodium's Valence Electrons: A Deep Dive into Atomic Structure and Reactivity
Sodium (Na), a ubiquitous element found in table salt and essential for human life, possesses a fascinating atomic structure that dictates its chemical behavior. Understanding its valence electrons is key to unlocking this behavior. This article delves deep into the world of sodium's valence electrons, exploring its electronic configuration, its impact on reactivity, and its broader significance in chemistry and beyond.
What are Valence Electrons?
Before we zero in on sodium, let's establish a firm understanding of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. They are the key players in chemical bonding, determining an atom's reactivity and the types of bonds it can form. The number of valence electrons significantly influences an atom's properties, including its electronegativity, ionization energy, and the types of compounds it forms.
The significance of valence electrons cannot be overstated: they dictate how an atom interacts with other atoms, forming the basis of all chemical reactions. Atoms strive for stability, often achieved by having a full outermost electron shell. This drive for stability is the driving force behind chemical bonding.
Determining Sodium's Valence Electrons
Sodium, with an atomic number of 11, has 11 protons and 11 electrons in a neutral atom. To determine its valence electrons, we need to understand its electronic configuration. This configuration describes how electrons are distributed among the different energy levels or shells within the atom.
Electronic Configuration of Sodium
The electronic configuration of sodium is 1s²2s²2p⁶3s¹. Let's break this down:
- 1s²: Two electrons in the first energy level (n=1), occupying the 's' subshell.
- 2s²: Two electrons in the second energy level (n=2), occupying the 's' subshell.
- 2p⁶: Six electrons in the second energy level (n=2), occupying the 'p' subshell.
- 3s¹: One electron in the third energy level (n=3), occupying the 's' subshell.
This configuration shows that sodium has three energy levels occupied by electrons. The outermost energy level (n=3) contains only one electron. Therefore, sodium has one valence electron.
The Impact of One Valence Electron on Sodium's Reactivity
The presence of just one valence electron profoundly impacts sodium's chemical behavior. Atoms tend to react in ways that achieve a stable electron configuration, often resembling the noble gases (Group 18 elements) with their full outermost shells. Sodium, with its single valence electron, readily loses this electron to achieve the stable electron configuration of neon (1s²2s²2p⁶).
This electron loss results in the formation of a sodium ion (Na⁺), a positively charged ion because it has lost a negatively charged electron. This process is known as ionization. The ionization energy of sodium is relatively low, meaning it requires relatively little energy to remove the single valence electron.
Sodium's Reactions: A Consequence of its Valence Electron
Because of its eagerness to lose its single valence electron, sodium is highly reactive, particularly with nonmetals. Let's explore some key reactions:
Reaction with Water
The reaction of sodium with water is famously vigorous. The sodium atom readily donates its valence electron to a water molecule, forming a sodium ion and hydroxide ions (OH⁻). This reaction generates hydrogen gas (H₂) and releases significant heat, often causing the hydrogen to ignite. The equation for this reaction is:
2Na(s) + 2H₂O(l) → 2NaOH(aq) + H₂(g)
The violence of this reaction underscores sodium's strong tendency to lose its valence electron and achieve a stable electronic configuration.
Reaction with Halogens
Sodium reacts readily with halogens (Group 17 elements like chlorine, bromine, and iodine) to form ionic compounds known as halides. For example, the reaction with chlorine produces sodium chloride (NaCl), commonly known as table salt. In this reaction, sodium loses its valence electron to chlorine, which gains it to complete its outermost shell. The resulting ions are held together by strong electrostatic forces, forming the ionic crystal lattice of sodium chloride. The equation is:
2Na(s) + Cl₂(g) → 2NaCl(s)
Reaction with Oxygen
Sodium reacts with oxygen in the air to form sodium oxide (Na₂O). Again, this reaction involves the loss of sodium's valence electron to oxygen. The equation is:
4Na(s) + O₂(g) → 2Na₂O(s)
However, this reaction is often less dramatic than the reaction with water or chlorine, as the oxide layer formed can act as a protective barrier.
Sodium's Valence Electron and its Properties
The single valence electron is responsible for many of sodium's physical and chemical properties:
- Low Ionization Energy: The ease with which sodium loses its valence electron contributes to its low ionization energy.
- Low Electronegativity: Sodium has a low electronegativity, meaning it has a weak attraction for electrons in a chemical bond. It prefers to donate its electron rather than share or gain one.
- Metallic Character: Sodium is a highly reactive metal, exhibiting characteristic metallic properties like conductivity (electrical and thermal) and malleability. The loosely held valence electrons contribute to this conductivity.
- Reactivity: As previously discussed, the single valence electron is responsible for sodium's high reactivity with other elements.
Sodium's Importance in Biological Systems
Sodium's role extends far beyond its presence in table salt. It plays a crucial role in various biological systems:
- Nerve Impulse Transmission: Sodium ions (Na⁺) are essential for the transmission of nerve impulses. The movement of sodium ions across cell membranes generates electrical signals that allow for communication between nerve cells.
- Muscle Contraction: Similar to nerve impulse transmission, sodium ions are critical for muscle contraction. The influx of sodium ions into muscle cells triggers the process of muscle contraction.
- Fluid Balance: Sodium helps regulate fluid balance in the body, ensuring proper hydration and preventing dehydration.
- Nutrient Absorption: Sodium aids in the absorption of certain nutrients in the digestive system.
The balance of sodium ions in the body is precisely regulated, and imbalances can lead to serious health problems.
Beyond the Basics: Exploring Advanced Concepts
Understanding sodium's valence electron is foundational to comprehending more advanced concepts in chemistry and physics:
- Band Theory of Solids: In solid sodium, the valence electrons are delocalized, forming a "sea" of electrons that contribute to the metal's high electrical conductivity.
- Photoelectric Effect: Sodium's low ionization energy makes it susceptible to the photoelectric effect, where electrons are emitted when light of sufficient frequency strikes the metal's surface.
- Spectroscopy: Analyzing the emission spectrum of sodium reveals information about the energy levels of its electrons, including its valence electron.
Conclusion: The Single Electron's Mighty Influence
Sodium's single valence electron is not just a minor detail; it is the architect of its chemical behavior, its reactivity, and its vital role in biological processes. Understanding this seemingly simple aspect of its atomic structure unlocks a wealth of knowledge about its properties, reactions, and importance in the world around us. From the vigorous reaction with water to its essential role in our bodies, the influence of sodium's single valence electron is undeniable and far-reaching. This exploration serves as a reminder of the fundamental importance of atomic structure in determining the properties and reactivity of elements and their compounds. The seemingly simple concept of valence electrons unlocks a deeper understanding of the complex world of chemistry and its impact on our lives.
Latest Posts
Latest Posts
-
What Is The Least Common Multiple Of 14 And 10
Mar 28, 2025
-
Is Water Condensing Endothermic Or Exothermic
Mar 28, 2025
-
Sum Of Exterior Angles Of A Heptagon
Mar 28, 2025
-
Which Shape Is Not A Parallelogram
Mar 28, 2025
-
Is 5 7 A Rational Number
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons For Sodium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
