Number Of Electrons In A 2p Orbital
listenit
Mar 16, 2025 · 6 min read
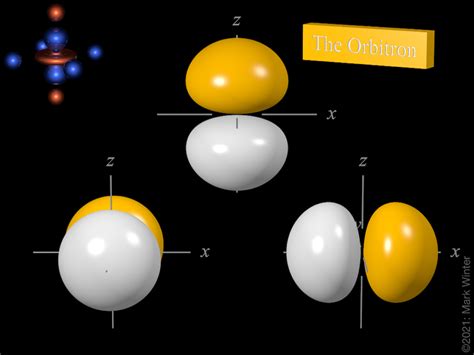
Table of Contents
Delving Deep: Understanding the Number of Electrons in a 2p Orbital
The seemingly simple question, "How many electrons can a 2p orbital hold?" opens a fascinating window into the quantum world of atomic structure. While the answer itself is straightforward, understanding why that number is the answer requires exploring fundamental concepts in chemistry and physics. This article dives deep into the intricacies of atomic orbitals, focusing specifically on the 2p orbital and its electron capacity. We'll cover the basics of quantum numbers, the shapes of atomic orbitals, the Pauli Exclusion Principle, and Hund's rule, all crucial to grasping the electron configuration of atoms.
Understanding Atomic Orbitals: A Foundation
Before tackling the 2p orbital, let's establish a solid base. Atomic orbitals are regions of space around an atom's nucleus where there's a high probability of finding an electron. These orbitals are not sharply defined boundaries; rather, they represent probability distributions. The behavior of electrons within these orbitals is governed by the principles of quantum mechanics, described by a set of quantum numbers.
Quantum Numbers: Defining an Electron's Address
Four quantum numbers describe each electron within an atom:
-
Principal Quantum Number (n): This number defines the electron shell or energy level. It's a positive integer (n = 1, 2, 3...). Higher values of 'n' indicate higher energy levels and greater distance from the nucleus. For the 2p orbital, n = 2, meaning it's in the second electron shell.
-
Azimuthal Quantum Number (l): This number defines the subshell or orbital type within a shell. It ranges from 0 to n-1. For example:
- l = 0 corresponds to an s orbital (spherical shape).
- l = 1 corresponds to a p orbital (dumbbell shape).
- l = 2 corresponds to a d orbital (more complex shapes).
- l = 3 corresponds to an f orbital (even more complex shapes).
In the case of the 2p orbital, l = 1, indicating a p subshell.
-
Magnetic Quantum Number (ml): This number defines the specific orbital within a subshell. It ranges from -l to +l, including 0. For a p subshell (l = 1), ml can be -1, 0, or +1. This means there are three 2p orbitals within the second electron shell: 2px, 2py, and 2pz. These orbitals are oriented along the x, y, and z axes, respectively.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum, or "spin," of the electron. It can have only two values: +1/2 (spin up, denoted as ↑) or -1/2 (spin down, denoted as ↓).
Visualizing the 2p Orbital: Shape and Orientation
Unlike the spherical s orbitals, p orbitals have a dumbbell shape. The three 2p orbitals (2px, 2py, and 2pz) are oriented along the x, y, and z axes, respectively, creating a three-dimensional arrangement around the nucleus. This orientation is crucial for understanding chemical bonding and molecular geometry. The electron density is highest along the axes, with a node (a region of zero electron density) at the nucleus.
The Pauli Exclusion Principle: A Capacity Limit
The Pauli Exclusion Principle is a cornerstone of quantum mechanics, stating that no two electrons in an atom can have the same set of four quantum numbers. This principle directly dictates the maximum number of electrons that can occupy an orbital.
Since each electron has a unique combination of n, l, ml, and ms, and each 2p orbital is defined by specific values of n, l, and ml, a maximum of two electrons can occupy each 2p orbital. One electron has spin up (+1/2), and the other has spin down (-1/2). This means each of the three 2p orbitals (2px, 2py, and 2pz) can hold a maximum of two electrons.
Hund's Rule: Filling Orbitals Efficiently
Hund's rule comes into play when filling orbitals within a subshell. It states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion, resulting in a more stable electron configuration. So, when filling the three 2p orbitals, each orbital will first receive one electron before any orbital receives a second electron.
The Final Answer: A Maximum of Six Electrons
Combining the Pauli Exclusion Principle and Hund's Rule, we can definitively answer the question: a 2p subshell, consisting of three 2p orbitals, can hold a maximum of six electrons (two electrons per orbital). This is a crucial piece of information for understanding the electron configurations of various atoms and their chemical behavior. Atoms with partially filled 2p orbitals are often highly reactive, readily participating in chemical reactions to achieve a more stable electron configuration.
Applications and Implications
Understanding the electron capacity of the 2p orbital has wide-ranging implications across various fields:
-
Chemical Bonding: The number of electrons in the 2p orbitals directly influences how atoms form chemical bonds. For example, the ability of carbon to form four bonds is a direct consequence of its four valence electrons (2s²2p²), with the 2p electrons participating in covalent bonding.
-
Spectroscopy: The electron transitions between different energy levels, including those within the 2p orbitals, give rise to characteristic spectral lines. This information is used in various analytical techniques, like atomic absorption spectroscopy (AAS) and atomic emission spectroscopy (AES).
-
Materials Science: The electronic properties of materials are profoundly affected by the electronic configurations of their constituent atoms, with the 2p electrons playing a significant role in many cases. This understanding is vital in the design and development of new materials with tailored properties.
-
Catalysis: Many catalysts rely on the presence of unpaired electrons in the 2p orbitals of transition metal atoms. These unpaired electrons participate in redox reactions, accelerating the catalytic process.
Beyond the Basics: More Advanced Considerations
While the basic concept of six electrons in the 2p subshell is straightforward, a deeper dive into quantum mechanics reveals more subtle nuances:
-
Orbital Hybridization: In many molecules, atomic orbitals can hybridize, meaning they combine to form new hybrid orbitals with different shapes and energies. This process significantly affects the spatial distribution of electrons and influences molecular properties.
-
Electron Correlation: Electron correlation refers to the influence of one electron's motion on the motion of another. This complex interaction is particularly significant in multi-electron atoms and requires advanced computational techniques to model accurately.
-
Relativistic Effects: In heavier atoms, relativistic effects become increasingly important. These effects arise from the high speeds of inner-shell electrons and can significantly alter energy levels and orbital shapes.
Conclusion: A Fundamental Concept in Chemistry
The seemingly simple question of how many electrons a 2p orbital can hold opens a rich exploration into the quantum mechanical world of atoms and molecules. The answer, six electrons (two in each of the three 2p orbitals), is a fundamental concept underpinning a vast range of chemical phenomena. Understanding this concept, along with the underlying principles of quantum numbers, the Pauli Exclusion Principle, and Hund's Rule, is essential for anyone seeking a deeper understanding of chemistry and its applications. From simple chemical bonds to complex material properties, the electron configuration of atoms, and specifically the occupancy of the 2p orbitals, plays a crucial role in shaping the world around us. The journey from a simple question to a deep understanding highlights the beauty and complexity of the quantum world.
Latest Posts
Latest Posts
-
Find Polar Coordinates Of The Point That Has Rectangular Coordinates
Mar 16, 2025
-
What Is A 35 Out Of 50
Mar 16, 2025
-
Difference Between Interval And Set Notation
Mar 16, 2025
-
Difference Between Sn1 And Sn2 Reaction
Mar 16, 2025
-
How Many Neutrons Does Krypton Have
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Number Of Electrons In A 2p Orbital . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
