How Many Neutrons Does Krypton Have
listenit
Mar 16, 2025 · 5 min read
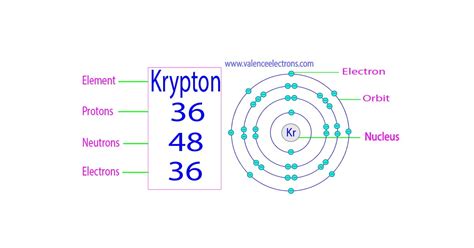
Table of Contents
How Many Neutrons Does Krypton Have? A Deep Dive into Isotopes and Nuclear Physics
Krypton, a noble gas with the symbol Kr and atomic number 36, doesn't have a single, fixed number of neutrons. This is because krypton exists in nature as a mixture of several isotopes. Understanding the number of neutrons in krypton requires delving into the fascinating world of isotopes and nuclear physics. This article will explore this topic comprehensively, covering the basics of atomic structure, isotopes, the specific isotopic composition of krypton, and the implications of neutron numbers in various applications.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we delve into the neutron count of krypton, let's refresh our understanding of the basic building blocks of an atom. Every atom is composed of three fundamental subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element; krypton, with 36 protons, is always krypton.
- Neutrons: Neutrally charged particles also residing in the nucleus. Unlike protons, the number of neutrons can vary within the same element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells. In a neutral atom, the number of electrons equals the number of protons.
Isotopes: The Key to Variable Neutron Numbers
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This difference in neutron number affects the atom's mass but not its chemical properties significantly. Isotopes are often represented as <sup>A</sup>X<sub>Z</sub>, where:
- X is the chemical symbol (e.g., Kr for krypton).
- Z is the atomic number (number of protons), which is always 36 for krypton.
- A is the mass number (number of protons + number of neutrons).
The mass number (A) directly indicates the total number of nucleons (protons and neutrons) in the nucleus. Therefore, to find the number of neutrons in a specific krypton isotope, we simply subtract the atomic number (Z) from the mass number (A): Number of neutrons = A - Z.
Krypton's Isotopes: A Diverse Family
Krypton has a significant number of isotopes, both stable and radioactive. The stable isotopes are those that do not undergo radioactive decay. Let's examine some of the most prevalent krypton isotopes:
- Krypton-84 (<sup>84</sup>Kr): This is the most abundant isotope of krypton, comprising approximately 57% of naturally occurring krypton. It has 36 protons and 48 neutrons (84 - 36 = 48).
- Krypton-86 (<sup>86</sup>Kr): The second most abundant isotope, making up about 17% of naturally occurring krypton. It contains 36 protons and 50 neutrons (86 - 36 = 50).
- Krypton-82 (<sup>82</sup>Kr): A relatively abundant stable isotope with 36 protons and 46 neutrons (82 - 36 = 46).
- Krypton-83 (<sup>83</sup>Kr): Another stable isotope, less abundant than the previous ones, possessing 36 protons and 47 neutrons (83 - 36 = 47).
- Krypton-80 (<sup>80</sup>Kr): A stable isotope, less abundant than others, consisting of 36 protons and 44 neutrons.
- Krypton-78 (<sup>78</sup>Kr): A stable isotope with 36 protons and 42 neutrons.
Radioactive Isotopes: Besides the stable isotopes listed above, krypton also has several radioactive isotopes with varying half-lives. These radioactive isotopes have different numbers of neutrons leading to nuclear instability. These isotopes are typically produced artificially and are used in specific applications, but their presence in natural krypton is negligible.
Calculating Neutron Numbers for Specific Krypton Isotopes
Let's illustrate the calculation of neutron numbers with a few examples:
- Krypton-84 (<sup>84</sup>Kr): Neutrons = 84 (mass number) - 36 (atomic number) = 48 neutrons
- Krypton-86 (<sup>86</sup>Kr): Neutrons = 86 - 36 = 50 neutrons
- Krypton-78 (<sup>78</sup>Kr): Neutrons = 78 - 36 = 42 neutrons
You can apply this simple formula (A - Z) to determine the neutron count for any krypton isotope, given its mass number (A).
The Significance of Neutron Numbers in Krypton's Properties
The number of neutrons in a krypton atom influences several of its properties:
- Nuclear Stability: The ratio of protons to neutrons significantly impacts an atom's nuclear stability. Isotopes with neutron-to-proton ratios that deviate significantly from the stability zone tend to be radioactive and undergo decay.
- Mass: The number of neutrons directly affects the atom's mass. Heavier isotopes have more neutrons and thus have higher atomic masses.
- Nuclear Reactions: The neutron number can influence how an isotope participates in nuclear reactions, including fission and fusion processes.
Applications of Krypton Isotopes
Krypton isotopes, particularly the stable ones, find various applications in different fields:
- Lighting: Krypton gas is used in certain types of lighting, including fluorescent lamps and high-intensity discharge lamps, due to its unique spectral properties.
- Medicine: Some krypton isotopes are employed in medical imaging techniques.
- Laser Technology: Krypton's isotopes play a role in specific types of lasers used in scientific and industrial applications.
- Scientific Research: Krypton's isotopic composition is a subject of scientific research to understand atmospheric processes and dating techniques.
Conclusion: A Variable Neutron Count Defines Krypton's Isotopic Landscape
To answer the question "How many neutrons does krypton have?", the correct response is not a single number but a range, depending on the specific isotope considered. Krypton exists as a mixture of several isotopes, each possessing a different number of neutrons. Understanding this isotopic diversity is crucial for appreciating the varied properties and applications of krypton in different fields of science and technology. The number of neutrons is critical to nuclear stability, impacting the radioactive or stable nature of each krypton isotope. This comprehensive exploration into the nuclear physics of krypton illuminates its unique characteristics and its essential role in various applications across numerous scientific domains. Further research into the specific properties of each isotope continues to reveal a deeper understanding of this fascinating noble gas.
Latest Posts
Latest Posts
-
Integrate 1 X 2 X 1
Mar 17, 2025
-
How Do Organisms Form Carbon Films
Mar 17, 2025
-
Compare And Contrast Acids And Bases
Mar 17, 2025
-
Cos Alpha Beta Cos Alpha Beta
Mar 17, 2025
-
2 8 N 4 N 4
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Does Krypton Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
