Compare And Contrast Acids And Bases
listenit
Mar 17, 2025 · 7 min read
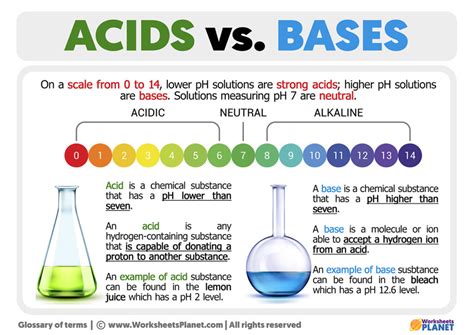
Table of Contents
Acids and Bases: A Comprehensive Comparison
Acids and bases are fundamental concepts in chemistry, playing crucial roles in countless natural processes and industrial applications. Understanding their properties, reactions, and differences is essential for anyone studying chemistry or related fields. This comprehensive guide will delve into the intricacies of acids and bases, comparing and contrasting their characteristics through various definitions and theories.
Defining Acids and Bases: A Multifaceted Approach
The classification of substances as acids or bases has evolved over time, with several prominent definitions offering unique perspectives. Let's explore the three most commonly used definitions: Arrhenius, Brønsted-Lowry, and Lewis.
Arrhenius Definition: The Foundation
The Arrhenius definition, proposed by Svante Arrhenius in 1884, is the simplest and historically the first. It defines acids as substances that increase the concentration of hydrogen ions (H⁺) in aqueous solution, while bases are substances that increase the concentration of hydroxide ions (OH⁻) in aqueous solution.
- Example of an Arrhenius acid: Hydrochloric acid (HCl) dissociates in water to form H⁺ and Cl⁻ ions.
- Example of an Arrhenius base: Sodium hydroxide (NaOH) dissociates in water to form Na⁺ and OH⁻ ions.
Limitations of the Arrhenius Definition: This definition is limited because it only applies to aqueous solutions and doesn't account for substances that exhibit acidic or basic properties without the presence of H⁺ or OH⁻ ions.
Brønsted-Lowry Definition: A Broader Perspective
The Brønsted-Lowry definition, introduced independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, provides a more comprehensive approach. It defines acids as proton donors (H⁺) and bases as proton acceptors. This definition extends beyond aqueous solutions, encompassing reactions in other solvents or even in the gas phase.
- Example: In the reaction between HCl and H₂O, HCl acts as a Brønsted-Lowry acid (donating a proton) and H₂O acts as a Brønsted-Lowry base (accepting a proton). The resulting products are H₃O⁺ (hydronium ion) and Cl⁻.
Conjugate Acid-Base Pairs: A key concept in the Brønsted-Lowry theory is the formation of conjugate acid-base pairs. When an acid donates a proton, it forms its conjugate base, and when a base accepts a proton, it forms its conjugate acid. In the HCl and H₂O example, HCl and Cl⁻ form a conjugate acid-base pair, and H₂O and H₃O⁺ form another.
Lewis Definition: The Electron Pair Perspective
The Lewis definition, proposed by Gilbert N. Lewis in 1923, provides the broadest perspective on acids and bases. It defines acids as electron pair acceptors and bases as electron pair donors. This definition encompasses a wide range of reactions, including those that don't involve protons.
- Example: The reaction between boron trifluoride (BF₃) and ammonia (NH₃). BF₃ acts as a Lewis acid (accepting an electron pair from NH₃), and NH₃ acts as a Lewis base (donating an electron pair to BF₃).
The Hierarchy of Definitions: It's important to note the hierarchical relationship between these definitions. All Arrhenius acids and bases are also Brønsted-Lowry acids and bases, and all Brønsted-Lowry acids and bases are also Lewis acids and bases. However, the reverse is not necessarily true. The Lewis definition is the most inclusive, encompassing reactions beyond the scope of the other two definitions.
Properties of Acids and Bases: A Detailed Comparison
While the definitions provide a theoretical framework, the practical application often relies on observable properties. Acids and bases exhibit distinct characteristics that allow for their identification and differentiation.
Physical Properties: Distinguishing Features
Several physical properties help distinguish acids and bases:
| Property | Acids | Bases |
|---|---|---|
| Taste | Sour | Bitter |
| Feel | Can be corrosive, may cause burns | Slippery or soapy feel |
| pH | Less than 7 | Greater than 7 |
| Electrical Conductivity | Aqueous solutions usually conduct electricity | Aqueous solutions usually conduct electricity |
| Reaction with Metals | React with reactive metals (like Zn, Mg) to produce hydrogen gas | Generally don't react with metals |
| Reaction with Indicators | Turn blue litmus paper red | Turn red litmus paper blue |
Chemical Properties: Reactivity and Transformations
The chemical properties of acids and bases are central to their role in various reactions:
Acids:
- Neutralization Reactions: Acids react with bases to produce salt and water. This is an essential reaction in many chemical processes and in the human body to maintain pH balance.
- Reaction with Carbonates: Acids react with carbonates (like limestone) to produce carbon dioxide gas, water, and a salt. This reaction is used in various industrial applications and geological processes.
- Reaction with Metals: Reactive metals react with acids to produce hydrogen gas and a metal salt. This reaction is often used to generate hydrogen gas in the laboratory.
- Decomposition of Certain Salts: Some salts react with acids to form other compounds.
Bases:
- Neutralization Reactions: As mentioned earlier, bases react with acids to produce salt and water.
- Saponification: Bases react with fats and oils to produce soap and glycerol. This is an important process in soap making.
- Reaction with Ammonia Salts: Bases react with ammonium salts to liberate ammonia gas, a pungent-smelling gas.
- Hydrolysis of Esters: Bases hydrolyze esters to form an alcohol and a carboxylic acid.
Strength of Acids and Bases: A Quantitative Measure
Acids and bases aren't all created equal. Their strength refers to the extent to which they dissociate or ionize in solution.
Strong Acids and Bases: Complete Dissociation
Strong acids and strong bases completely dissociate into ions in aqueous solutions. This means that virtually all of the acid or base molecules break apart into their constituent ions.
- Examples of strong acids: HCl, HBr, HI, HNO₃, H₂SO₄ (first dissociation only), HClO₄.
- Examples of strong bases: NaOH, KOH, LiOH, Ca(OH)₂, Ba(OH)₂.
Weak Acids and Bases: Partial Dissociation
Weak acids and weak bases only partially dissociate in aqueous solutions. This means that only a small fraction of the acid or base molecules break apart into their constituent ions. The equilibrium between the undissociated molecules and the ions plays a crucial role in determining their properties.
- Examples of weak acids: Acetic acid (CH₃COOH), carbonic acid (H₂CO₃), formic acid (HCOOH).
- Examples of weak bases: Ammonia (NH₃), methylamine (CH₃NH₂), pyridine (C₅H₅N).
Measuring Acid and Base Strength: pKa and pKb
The strength of weak acids and bases is quantitatively expressed using pKa and pKb values, which are derived from the acid dissociation constant (Ka) and base dissociation constant (Kb), respectively. Lower pKa values indicate stronger acids, while lower pKb values indicate stronger bases.
Applications of Acids and Bases: A Wide Spectrum
Acids and bases are ubiquitous, finding applications in countless areas, from everyday life to cutting-edge technologies.
Everyday Applications: A Closer Look
- Food and Beverages: Many foods and drinks contain acids (e.g., citric acid in citrus fruits, acetic acid in vinegar) or bases (e.g., baking soda, which is a base).
- Cleaning Products: Many cleaning products utilize acidic or basic solutions to remove dirt and grime. Acidic cleaners are often used for removing mineral deposits, while basic cleaners are used for degreasing.
- Pharmaceuticals: Many drugs and medicines involve acidic or basic compounds that interact with the body to produce their therapeutic effects.
- Digestion: The human digestive system relies on acids (e.g., hydrochloric acid in the stomach) and bases (e.g., bicarbonate ions in the pancreas) to break down food.
Industrial Applications: Large-Scale Uses
- Chemical Synthesis: Acids and bases are essential reagents in countless chemical reactions, serving as catalysts, solvents, and reactants in the production of various chemicals.
- Materials Science: Acids and bases play critical roles in the processing and treatment of various materials, including metals, polymers, and ceramics.
- Environmental Remediation: Acids and bases are used in various environmental remediation processes, such as neutralizing acid rain or treating contaminated water.
- Energy Production: Acid-base reactions play a role in certain types of batteries and fuel cells.
Conclusion: A Unified Understanding
The study of acids and bases reveals a fascinating interplay of theoretical concepts and practical applications. From the simple Arrhenius definition to the comprehensive Lewis theory, our understanding of these fundamental chemical entities has evolved significantly. Their diverse properties and wide-ranging applications highlight their indispensable role in chemistry, biology, and numerous industrial processes. A strong grasp of acid-base chemistry is crucial for anyone seeking to delve deeper into the scientific world.
Latest Posts
Latest Posts
-
1 2 2 3 3 4
Mar 17, 2025
-
4 Liters Is How Many Ml
Mar 17, 2025
-
Least Common Factor Of 8 And 10
Mar 17, 2025
-
Why Is Dna Called The Blueprint
Mar 17, 2025
-
If Sin Theta Is 4 5 What Is Cos Theta
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Compare And Contrast Acids And Bases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
