Difference Between Sn1 And Sn2 Reaction
listenit
Mar 16, 2025 · 6 min read
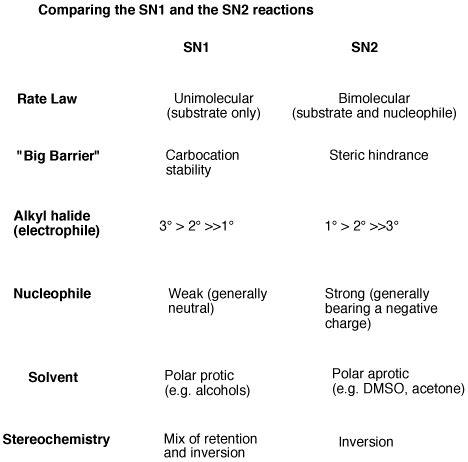
Table of Contents
The Great Divide: Understanding the Differences Between SN1 and SN2 Reactions
Organic chemistry can feel like navigating a labyrinth, especially when confronted with the intricacies of nucleophilic substitution reactions. Two prominent players in this complex world are SN1 and SN2 reactions. While both involve the substitution of one nucleophile for another on a carbon atom, their mechanisms, reaction rates, stereochemistry, and substrate preferences differ significantly. This comprehensive guide will delve into the core distinctions between SN1 and SN2 reactions, providing a clear understanding of their unique characteristics.
Understanding Nucleophilic Substitution Reactions
Before diving into the differences, let's establish a common ground. Both SN1 and SN2 reactions are types of nucleophilic substitution, meaning they involve a nucleophile (a species with a lone pair of electrons seeking a positive charge) attacking an electrophilic carbon atom (a carbon atom with a partial or full positive charge) and displacing a leaving group. The leaving group is an atom or group of atoms that departs with a pair of electrons. Common examples include halides (Cl⁻, Br⁻, I⁻), water (H₂O), and tosylate (OTs).
SN1 Reactions: A Two-Step Process
SN1 reactions, or unimolecular nucleophilic substitution reactions, proceed through a two-step mechanism. The rate-determining step (the slowest step) involves the formation of a carbocation intermediate.
Step 1: Formation of the Carbocation Intermediate
This step involves the departure of the leaving group from the substrate. The bond between the carbon and the leaving group breaks heterolytically, meaning the electrons in the bond go entirely to the leaving group, resulting in a positively charged carbon atom – the carbocation. The stability of this carbocation is crucial in determining the rate of the reaction. Tertiary carbocations (R₃C⁺) are the most stable, followed by secondary (R₂CH⁺), and primary carbocations (RCH₂⁺) are the least stable. Methyl carbocations (CH₃⁺) are extremely unstable.
Step 2: Nucleophilic Attack
In the second step, the nucleophile attacks the carbocation. Since the carbocation is planar, the nucleophile can attack from either side, leading to a racemic mixture of products (a 50:50 mixture of enantiomers, if the starting material is chiral). This lack of stereospecificity is a key characteristic of SN1 reactions.
Factors Affecting SN1 Reaction Rates
Several factors influence the rate of an SN1 reaction:
- Substrate Structure: Tertiary substrates react fastest, followed by secondary, and primary substrates are extremely slow or unreactive. This is directly tied to the stability of the carbocation intermediate.
- Leaving Group Ability: Good leaving groups, such as I⁻, Br⁻, and Cl⁻, stabilize the negative charge well, facilitating their departure and accelerating the reaction. Poor leaving groups, such as OH⁻ and NH₂, hinder the reaction. Conversion of poor leaving groups into better leaving groups, often using protonation or tosylation, is often necessary.
- Solvent: Polar protic solvents (solvents with an O-H or N-H bond, such as water or alcohols) are preferred for SN1 reactions. These solvents stabilize both the carbocation intermediate and the leaving group, facilitating the reaction.
- Nucleophile Concentration: The concentration of the nucleophile doesn't affect the rate of the reaction because the nucleophile participates in the second, faster step. This is why it's called a unimolecular reaction.
SN2 Reactions: A Concerted Mechanism
SN2 reactions, or bimolecular nucleophilic substitution reactions, proceed through a concerted mechanism, meaning the bond breaking and bond forming occur simultaneously in a single step.
The Concerted Mechanism
In an SN2 reaction, the nucleophile attacks the carbon atom bearing the leaving group from the backside (opposite side of the leaving group). This backside attack causes the inversion of configuration at the stereocenter (if present). This inversion is known as Walden inversion. The transition state involves a five-coordinate carbon atom, where the nucleophile and the leaving group are partially bonded to the carbon.
Factors Affecting SN2 Reaction Rates
The rate of an SN2 reaction depends on several factors:
- Substrate Structure: Methyl and primary substrates react fastest. Secondary substrates react slower, and tertiary substrates are essentially unreactive. The steric hindrance around the carbon atom plays a crucial role. Bulky groups hinder the backside attack by the nucleophile.
- Leaving Group Ability: Good leaving groups facilitate the reaction. The same principles apply as in SN1 reactions.
- Nucleophile Strength: Stronger nucleophiles react faster. Strong nucleophiles have a high electron density and readily donate electrons. The concentration of the nucleophile also directly impacts the rate of reaction.
- Solvent: Polar aprotic solvents (solvents without an O-H or N-H bond, such as DMSO or acetone) are preferred for SN2 reactions. These solvents solvate the cations well, leaving the nucleophile less solvated and more reactive.
Key Differences Between SN1 and SN2 Reactions: A Summary Table
| Feature | SN1 Reaction | SN2 Reaction |
|---|---|---|
| Mechanism | Two-step: carbocation intermediate | Concerted: one-step |
| Rate-determining step | Carbocation formation | Nucleophilic attack |
| Rate law | Rate = k[substrate] | Rate = k[substrate][nucleophile] |
| Substrate | Tertiary > Secondary > Primary (Primary is very slow) | Methyl > Primary > Secondary (Tertiary is very slow) |
| Stereochemistry | Racemization (loss of chirality) | Inversion of configuration (Walden inversion) |
| Nucleophile | Weak or strong nucleophiles can be used | Strong nucleophiles are preferred |
| Leaving Group | Good leaving groups are essential | Good leaving groups are essential |
| Solvent | Polar protic solvents are preferred | Polar aprotic solvents are preferred |
Predicting the Mechanism: A Practical Approach
Determining whether a reaction will proceed via SN1 or SN2 depends on a combination of factors. Generally:
- Tertiary substrates favor SN1 reactions.
- Primary substrates favor SN2 reactions.
- Secondary substrates can undergo both SN1 and SN2 reactions depending on the nucleophile, leaving group, and solvent. Strong nucleophiles in polar aprotic solvents generally favor SN2, while weak nucleophiles in polar protic solvents favor SN1.
Analyzing the specific conditions of the reaction is crucial for predicting the dominant mechanism.
Advanced Considerations: Competing Reactions
It's important to remember that SN1 and SN2 reactions aren't always mutually exclusive. In certain cases, both mechanisms might compete, leading to a mixture of products. Other competing reactions, such as elimination reactions (E1 and E2), can also occur, particularly at higher temperatures or with strong bases. Understanding the factors that influence each reaction type is essential for predicting the outcome and optimizing the reaction conditions to favor the desired product.
Conclusion: Mastering the Nuances of Nucleophilic Substitution
Understanding the differences between SN1 and SN2 reactions is fundamental to mastering organic chemistry. By carefully considering the substrate structure, nucleophile strength, leaving group ability, and solvent effects, one can predict the dominant mechanism and optimize reaction conditions to achieve the desired outcome. The intricacies of these reactions highlight the elegance and complexity of organic chemistry, making it a fascinating area of study for aspiring chemists and researchers alike. This detailed comparison serves as a strong foundation for further exploration into the world of organic reaction mechanisms. Continued practice and problem-solving are key to truly mastering these concepts.
Latest Posts
Latest Posts
-
What Is The Lcm Of 5 And 7
Mar 17, 2025
-
Integrate 1 X 2 X 1
Mar 17, 2025
-
How Do Organisms Form Carbon Films
Mar 17, 2025
-
Compare And Contrast Acids And Bases
Mar 17, 2025
-
Cos Alpha Beta Cos Alpha Beta
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Sn1 And Sn2 Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
