Molarity Of Acetic Acid In Vinegar
listenit
Mar 25, 2025 · 6 min read
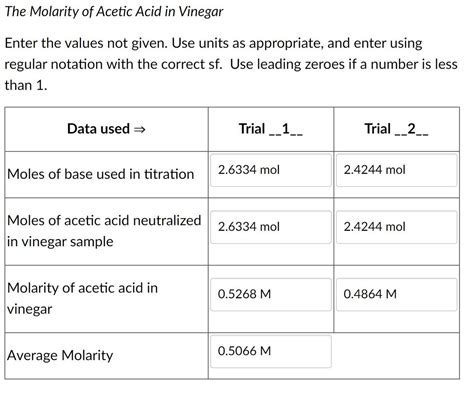
Table of Contents
Determining the Molarity of Acetic Acid in Vinegar: A Comprehensive Guide
Vinegar, a staple in kitchens worldwide, is essentially a dilute solution of acetic acid in water. Understanding the molarity of acetic acid in vinegar – the concentration of acetic acid molecules per liter of solution – is crucial for various applications, from culinary uses to scientific experiments. This comprehensive guide delves into the methods for determining this molarity, exploring the underlying chemistry and practical considerations involved.
Understanding Molarity and its Significance
Molarity (M) is a fundamental unit of concentration in chemistry, defined as the number of moles of solute (in this case, acetic acid) per liter of solution. A 1 M solution contains one mole of solute per liter of solution. Knowing the molarity of acetic acid in vinegar allows us to:
- Control reactions: In chemical reactions involving vinegar, the precise concentration of acetic acid is vital for predictable outcomes. A known molarity ensures consistent and reproducible results.
- Standardize solutions: Vinegar can be used to standardize solutions of bases, a crucial step in many analytical procedures. This requires an accurate determination of the vinegar's acetic acid molarity.
- Quality control: The molarity of acetic acid is an indicator of vinegar quality. Variations from expected concentrations may signal inconsistencies in production or adulteration.
- Culinary applications: While less precise, understanding molarity can help in recipe adjustments where the acidity of vinegar is a critical factor.
Methods for Determining the Molarity of Acetic Acid in Vinegar
Several methods can determine the molarity of acetic acid in vinegar. The most common and widely accessible method involves titration.
Titration: A Precise Method
Titration is a quantitative analytical technique where a solution of known concentration (the titrant) is reacted with a solution of unknown concentration (the analyte) until the reaction is complete. In determining the molarity of acetic acid in vinegar, a standardized base, usually sodium hydroxide (NaOH), is used as the titrant.
The Chemistry Behind the Titration:
Acetic acid (CH₃COOH) is a weak monoprotic acid, meaning it donates one proton (H⁺) per molecule. Sodium hydroxide (NaOH) is a strong base, readily dissociating into Na⁺ and OH⁻ ions. The reaction between them is a neutralization reaction:
CH₃COOH(aq) + NaOH(aq) → CH₃COONa(aq) + H₂O(l)
This reaction proceeds until all the acetic acid in the vinegar sample has reacted with the sodium hydroxide. The point at which this occurs is called the equivalence point.
Procedure:
- Preparation: Accurately weigh a known volume of vinegar using a volumetric pipette and transfer it to a clean Erlenmeyer flask. Add a few drops of a suitable indicator, such as phenolphthalein. Phenolphthalein is colorless in acidic solutions and turns pink in basic solutions.
- Titration: Fill a burette with a standardized NaOH solution of known molarity. Slowly add the NaOH solution to the vinegar sample while swirling the flask continuously. The solution will initially remain colorless. As the equivalence point approaches, the solution will begin to turn a faint pink.
- Equivalence Point: Continue adding NaOH dropwise until a persistent faint pink color persists for at least 30 seconds. This indicates that the equivalence point has been reached. Record the volume of NaOH solution used.
- Calculations: Using the stoichiometry of the balanced chemical equation and the known molarity and volume of NaOH used, the moles of NaOH reacted can be calculated. Since the mole ratio of acetic acid to NaOH is 1:1, this is also the number of moles of acetic acid in the vinegar sample. Finally, dividing the moles of acetic acid by the volume of vinegar (in liters) gives the molarity of acetic acid.
Example Calculation:
Let's say 25.00 mL of vinegar was titrated with 20.00 mL of 0.100 M NaOH.
- Moles of NaOH = (0.100 mol/L) * (0.02000 L) = 0.00200 mol
- Moles of CH₃COOH = 0.00200 mol (due to 1:1 stoichiometry)
- Molarity of CH₃COOH = (0.00200 mol) / (0.02500 L) = 0.0800 M
Therefore, the molarity of acetic acid in this vinegar sample is 0.0800 M.
Factors Affecting Accuracy
Several factors can affect the accuracy of the titration method:
- Indicator choice: The choice of indicator can slightly affect the endpoint determination. Phenolphthalein is a common choice, but others, such as methyl orange, could be used, potentially leading to slightly different results.
- Standardization of NaOH: The accuracy of the NaOH solution's molarity is critical. Any error in standardizing the NaOH solution will propagate through the calculations.
- Temperature: Temperature changes can affect the reaction rate and the volume measurements.
- Sample handling: Careful handling of the vinegar sample is essential to avoid contamination or loss of material.
Advanced Techniques
While titration is the most common method, other techniques can determine acetic acid molarity, offering greater precision or handling specific situations:
- Spectrophotometry: This technique measures the absorbance of light by the vinegar solution. The absorbance is directly proportional to the concentration of acetic acid. However, this method requires careful calibration and consideration of potential interferences from other components in the vinegar.
- Gas chromatography: This separates the components of the vinegar, allowing for precise quantification of acetic acid. This is a more sophisticated technique often used in research settings.
- Nuclear magnetic resonance (NMR) spectroscopy: NMR provides detailed information about the molecular structure and concentration of various components in the vinegar sample. This is a highly accurate method, often employed in research and quality control.
Practical Applications Beyond the Lab
Understanding the molarity of acetic acid in vinegar extends beyond scientific experimentation. Its applications range across numerous fields:
- Food science: Molarity plays a crucial role in developing recipes and standardizing food products that use vinegar as an ingredient. Specific concentrations of acetic acid influence the taste, texture, and shelf life of many food items.
- Biochemistry: Vinegar's acidic nature makes it useful in various biochemical applications, including pH control in biological experiments and as a cleaning agent in laboratory settings.
- Environmental science: Vinegar can be used in certain environmental remediation projects, particularly those involving the removal of mineral deposits or cleaning equipment. Accurate knowledge of acetic acid concentration is necessary for efficient and safe application.
Conclusion
Determining the molarity of acetic acid in vinegar is a valuable skill with practical implications across several disciplines. Titration, while requiring careful execution, provides a relatively simple and accessible method for achieving accurate results. Understanding the underlying chemistry, potential sources of error, and alternative techniques expands the capabilities for precise concentration determination, ultimately leading to more controlled and effective applications of vinegar in diverse fields. The importance of accurate molarity determination underlines the versatility and significance of this seemingly simple household ingredient. Further research and exploration into these techniques would enhance our understanding and efficient utilization of vinegar in various applications.
Latest Posts
Latest Posts
-
The Study Of The Cells Is Called
Mar 25, 2025
-
Can Mixtures Be Separated By Physical Means
Mar 25, 2025
-
Which State Of Matter Has The Highest Kinetic Energy
Mar 25, 2025
-
What Is The Opposite Of Sublimation
Mar 25, 2025
-
Square Root Of 1 Divided By 8
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Molarity Of Acetic Acid In Vinegar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
