Molar Mass Of Helium In Kg
listenit
Mar 19, 2025 · 4 min read
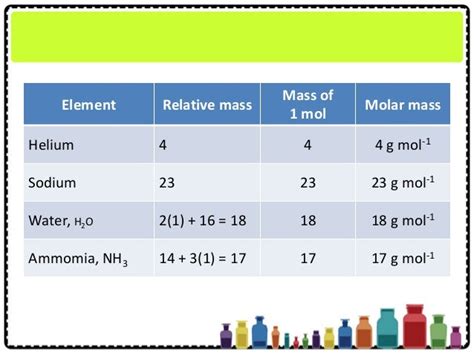
Table of Contents
Molar Mass of Helium in kg: A Comprehensive Guide
The molar mass of helium, a crucial parameter in various scientific and engineering applications, represents the mass of one mole of helium atoms. While often expressed in grams per mole (g/mol), understanding its equivalent in kilograms per mole (kg/mol) is essential for certain calculations, particularly those involving larger scales or requiring consistency with SI units. This comprehensive guide delves into the concept of molar mass, specifically focusing on helium's molar mass in kg, exploring its applications, and addressing potential confusion related to unit conversions.
Understanding Molar Mass
The molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry, defined as the amount of substance containing the same number of elementary entities (atoms, molecules, ions, etc.) as there are atoms in 12 grams of carbon-12. This number, known as Avogadro's number (N<sub>A</sub>), is approximately 6.022 x 10<sup>23</sup>.
Therefore, the molar mass connects the microscopic world of atoms and molecules to the macroscopic world of measurable quantities like mass and volume. It's a crucial bridge between the atomic mass unit (amu) and grams (or kilograms). The atomic mass of an element, usually found on the periodic table, is essentially the average mass of its isotopes, weighted by their abundance. This atomic mass, expressed in amu, is numerically equal to the molar mass expressed in g/mol.
Helium's Molar Mass: From g/mol to kg/mol
Helium (He), a noble gas renowned for its lightness and inertness, has an atomic mass of approximately 4.0026 amu. This means that the molar mass of helium is approximately 4.0026 g/mol. To convert this to kg/mol, we simply apply the standard metric conversion:
1 kg = 1000 g
Therefore, the molar mass of helium in kg/mol is:
4.0026 g/mol * (1 kg / 1000 g) = 0.0040026 kg/mol
This seemingly small value highlights helium's extremely low density, a property that contributes to its many uses.
Applications Requiring Helium's Molar Mass in kg/mol
While many calculations utilize the g/mol value, several applications benefit from expressing helium's molar mass in kg/mol:
1. Large-Scale Industrial Processes:
In industrial settings dealing with significant quantities of helium, using kg/mol provides a more manageable numerical scale. Calculations involving the mass of helium in large balloons, cryogenic systems, or industrial leak detection systems are simplified with this unit. For example, determining the total mass of helium used in a large-scale helium liquefaction plant is easier when using kg/mol.
2. Aerospace and Rocketry:
Helium is commonly used as a propellant or lifting gas in aerospace applications. Calculations relating to the buoyancy and lift capacity of helium-filled balloons or airships are often simplified by using kg/mol for mass calculations, aligning with the SI unit system commonly employed in engineering design.
3. Advanced Physics and Engineering Calculations:
In fields like nuclear fusion research or advanced materials science, where extremely precise calculations are paramount, utilizing kg/mol maintains consistency within the SI unit system, reducing the risk of unit conversion errors that can propagate through complex calculations.
4. Environmental and Atmospheric Science:
When studying atmospheric composition or helium leakage from geological formations, using kg/mol helps researchers maintain consistency in units, particularly when integrating data from diverse sources and instruments.
Why Understanding Unit Conversions is Crucial
The seemingly simple task of converting between g/mol and kg/mol underscores the importance of precise unit conversions in scientific and engineering work. A small mistake in unit conversion can lead to significant errors in calculations, potentially affecting experimental results, design parameters, or even safety protocols.
For instance, misinterpreting the molar mass of helium as 4.0026 kg/mol instead of 4.0026 g/mol would lead to a massive overestimation in mass calculations, resulting in inaccurate predictions and potentially disastrous consequences in applications like balloon design or cryogenic storage.
Always double-check your unit conversions and ensure consistency throughout your calculations.
Common Misconceptions and Clarifications
Several misunderstandings often arise concerning molar mass and its units:
-
Confusing Atomic Mass with Molar Mass: While numerically equivalent (in g/mol and amu respectively), they represent different concepts. Atomic mass refers to the mass of a single atom, while molar mass refers to the mass of a mole of atoms.
-
Ignoring Significant Figures: When performing calculations, maintain the appropriate number of significant figures to reflect the precision of the input data. Using too few significant figures can lead to inaccurate results.
-
Incorrect Unit Conversions: Always carefully track units during conversions. A common error is neglecting to consider the conversion factor (1000 g/kg) accurately.
Conclusion: The Importance of Precision
The molar mass of helium in kg/mol, while seemingly a minor variation from the commonly used g/mol, holds significant importance in various scientific and engineering contexts, particularly in large-scale applications and when adhering strictly to SI unit systems. Understanding this conversion, along with a thorough grasp of unit conversions in general, is crucial for accurate calculations and reliable results in any scientific or engineering endeavor. Accuracy and precision are not merely details but essential components of achieving reliable and safe outcomes. By paying close attention to these details, we ensure that our scientific and engineering endeavors are sound and successful.
Latest Posts
Latest Posts
-
Is 87 A Prime Or Composite Number
Mar 20, 2025
-
How Many Electrons Does P Have
Mar 20, 2025
-
Whats A 26 Out Of 30
Mar 20, 2025
-
Nucleic Acids Are Made Of Monomers Called
Mar 20, 2025
-
How Many Pints In 1 Lb
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Molar Mass Of Helium In Kg . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
