Nucleic Acids Are Made Of Monomers Called
listenit
Mar 20, 2025 · 6 min read
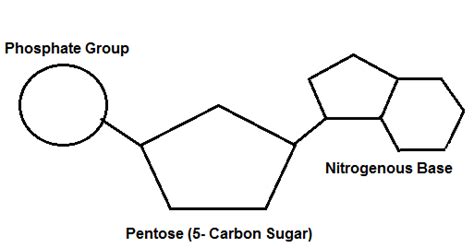
Table of Contents
Nucleic Acids Are Made of Monomers Called Nucleotides: A Deep Dive
Nucleic acids are fundamental macromolecules essential for life. They carry the genetic blueprint of all living organisms, directing cellular processes and heredity. Understanding their structure and function begins with knowing their building blocks: nucleotides. This article will delve deep into the world of nucleotides, exploring their composition, the different types, their role in forming nucleic acids (DNA and RNA), and their broader significance in biology.
The Building Blocks: Nucleotides Explained
Nucleotides are the monomers, or individual units, that polymerize to form the long chains of nucleic acids. Each nucleotide is composed of three essential components:
1. A Pentose Sugar: The Backbone's Foundation
The pentose sugar is a five-carbon sugar that forms the backbone of the nucleotide. There are two types of pentose sugars found in nucleic acids:
- Ribose: Found in ribonucleic acid (RNA). It has a hydroxyl (-OH) group attached to the 2' carbon atom.
- Deoxyribose: Found in deoxyribonucleic acid (DNA). It lacks a hydroxyl group at the 2' carbon atom, hence the "deoxy" prefix. This seemingly small difference has significant implications for the structure and stability of DNA compared to RNA.
The difference in the 2' carbon atom significantly impacts the structural stability and reactivity of the nucleic acid. The presence of the hydroxyl group in ribose makes RNA more reactive and less stable than DNA. This contributes to RNA’s diverse roles in various cellular processes, while DNA’s stability is crucial for its long-term storage of genetic information.
2. A Nitrogenous Base: Carrying Genetic Information
The nitrogenous base is a crucial component, responsible for carrying the genetic information. There are five main types of nitrogenous bases:
- Adenine (A): A purine base, characterized by a double-ring structure.
- Guanine (G): Another purine base, also with a double-ring structure.
- Cytosine (C): A pyrimidine base, having a single-ring structure.
- Thymine (T): A pyrimidine base found only in DNA.
- Uracil (U): A pyrimidine base found only in RNA, replacing thymine.
The specific sequence of these bases along the nucleic acid chain determines the genetic code. The pairing of bases through hydrogen bonds (A with T or U, and G with C) is fundamental to the double helix structure of DNA and the secondary structures of RNA.
3. A Phosphate Group: Linking the Units
The phosphate group (PO₄³⁻) is a negatively charged molecule. It links the 5' carbon of one pentose sugar to the 3' carbon of the next pentose sugar, creating a phosphodiester bond. This bond forms the sugar-phosphate backbone of the nucleic acid chain, with the nitrogenous bases projecting outward. The polarity of this backbone (5' to 3' directionality) is crucial for DNA replication and RNA transcription. The negative charge of the phosphate group also influences the overall properties of nucleic acids, including their solubility and interaction with proteins.
Nucleotide Formation and Nomenclature
Nucleotides are formed through a series of enzymatic reactions involving the attachment of the nitrogenous base to the pentose sugar, followed by the addition of the phosphate group. The resulting nucleoside monophosphate can then acquire additional phosphate groups to form nucleoside diphosphates (NDPs) and nucleoside triphosphates (NTPs). These triphosphates, such as ATP (adenosine triphosphate), GTP (guanosine triphosphate), CTP (cytidine triphosphate), and UTP (uridine triphosphate), are crucial energy carriers in cellular metabolism. They also serve as the building blocks for RNA synthesis. The corresponding deoxyribonucleotide triphosphates (dNTPs) are used in DNA synthesis.
The nomenclature of nucleotides is often complex but follows a systematic pattern. For example, adenosine monophosphate (AMP) is the nucleotide containing adenine, ribose, and one phosphate group. Adenosine diphosphate (ADP) has two phosphate groups, and adenosine triphosphate (ATP) has three. The "deoxy" prefix indicates the presence of deoxyribose instead of ribose. For instance, deoxyadenosine triphosphate (dATP) is a building block for DNA synthesis. This naming convention applies consistently across all the bases and phosphate numbers. Understanding this systematic nomenclature is vital for deciphering biochemical pathways and processes.
From Monomers to Polymers: The Formation of Nucleic Acids
Nucleotides assemble to form polynucleotide chains through the formation of phosphodiester bonds. The 3' hydroxyl group of one nucleotide reacts with the 5' phosphate group of the next nucleotide, releasing a water molecule and creating a phosphodiester linkage. This process continues, adding nucleotides to the growing chain in a 5' to 3' direction. The precise order of nucleotides in a nucleic acid chain dictates the genetic information it encodes.
DNA: The Double Helix
DNA, or deoxyribonucleic acid, typically exists as a double helix. Two polynucleotide chains are wound around each other, held together by hydrogen bonds between complementary base pairs: adenine (A) with thymine (T), and guanine (G) with cytosine (C). This double helix structure provides remarkable stability and a mechanism for precise replication. The antiparallel nature of the two strands (one running 5' to 3', the other 3' to 5') is critical for the mechanism of DNA replication. The major and minor grooves formed in the double helix also provide access points for DNA-binding proteins involved in gene regulation and DNA repair.
RNA: Diverse Structures and Functions
RNA, or ribonucleic acid, is typically single-stranded, but it can fold into complex secondary and tertiary structures due to base pairing within the molecule. This folding creates specific structural domains crucial for RNA's diverse roles:
- Messenger RNA (mRNA): Carries the genetic code from DNA to ribosomes for protein synthesis.
- Transfer RNA (tRNA): Carries amino acids to ribosomes during translation.
- Ribosomal RNA (rRNA): Forms part of the ribosome structure, essential for protein synthesis.
- MicroRNA (miRNA): Regulates gene expression by binding to mRNA molecules.
The versatility of RNA structure allows it to participate in a wide range of cellular processes, ranging from gene regulation to catalysis. This contrasts with DNA, whose primary function is long-term storage of genetic information.
The Significance of Nucleotides Beyond Nucleic Acids
The importance of nucleotides extends far beyond their role as the building blocks of nucleic acids. They are involved in numerous cellular processes:
- Energy Transfer: ATP (adenosine triphosphate) is the primary energy currency of the cell, powering many metabolic reactions.
- Signal Transduction: Cyclic AMP (cAMP) acts as a second messenger in various signaling pathways.
- Coenzyme Function: Nicotinamide adenine dinucleotide (NAD⁺) and flavin adenine dinucleotide (FAD) are essential coenzymes involved in redox reactions.
These diverse functions highlight the central importance of nucleotides in all aspects of cellular metabolism and regulation. They are not just the building blocks of genetic material; they are active participants in a vast array of biochemical processes that keep life going.
Conclusion: A Cornerstone of Life
In conclusion, nucleotides, composed of a pentose sugar, a nitrogenous base, and a phosphate group, are the fundamental monomers of nucleic acids – DNA and RNA. Their specific sequence determines the genetic information encoded within these molecules, which guide the processes of life. Beyond their structural role, nucleotides play critical roles in energy transfer, signaling pathways, and enzymatic reactions. A deep understanding of nucleotides and their properties is therefore fundamental to appreciating the complexity and intricacies of biological systems. Their significance extends to fields beyond basic biology, influencing areas like medicine, biotechnology, and genetic engineering. Future research into nucleotide chemistry and function continues to reveal new insights into the mechanisms that govern life itself. The continued exploration of these fascinating molecules will undoubtedly lead to breakthroughs in various scientific fields.
Latest Posts
Latest Posts
-
What Are The Products Of The Neutralization Reaction
Mar 20, 2025
-
Is A Rhombus A Regular Polygon
Mar 20, 2025
-
What Percentage Of 25 Is 4
Mar 20, 2025
-
How Much Is 200 Degrees Celsius
Mar 20, 2025
-
Square Root Of 3 Divided By 2
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Nucleic Acids Are Made Of Monomers Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
