What Are The Products Of The Neutralization Reaction
listenit
Mar 20, 2025 · 6 min read
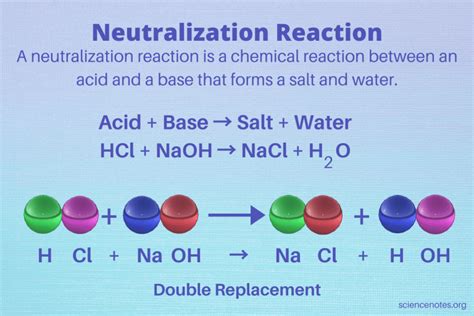
Table of Contents
What Are the Products of a Neutralization Reaction? A Comprehensive Guide
Neutralization reactions are fundamental chemical processes with far-reaching implications in various fields, from industrial manufacturing to biological systems. Understanding the products of these reactions is crucial for predicting reaction outcomes and controlling chemical processes. This comprehensive guide delves deep into the intricacies of neutralization reactions, exploring the types of reactants involved, the products formed, and the applications of these reactions in diverse contexts.
Understanding Neutralization Reactions: An Overview
A neutralization reaction, at its core, is a chemical reaction between an acid and a base. The defining characteristic of this reaction is the combination of hydrogen ions (H⁺) from the acid and hydroxide ions (OH⁻) from the base to form water (H₂O). This process effectively neutralizes the acidic and basic properties of the reactants, hence the name "neutralization." The other product formed is a salt, an ionic compound composed of the cation from the base and the anion from the acid.
The general equation for a neutralization reaction can be represented as:
Acid + Base → Salt + Water
This seemingly simple equation hides a wealth of complexity, as the specific products depend heavily on the strength and nature of the acid and base involved.
Types of Neutralization Reactions and Their Products
Neutralization reactions can be broadly classified based on the strength of the acid and base involved:
1. Strong Acid-Strong Base Neutralization
Reactions involving a strong acid (e.g., hydrochloric acid, HCl; sulfuric acid, H₂SO₄; nitric acid, HNO₃) and a strong base (e.g., sodium hydroxide, NaOH; potassium hydroxide, KOH; calcium hydroxide, Ca(OH)₂ ) are characterized by complete ionization of both reactants. This results in a complete neutralization, yielding water and a neutral salt.
Example:
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic example:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Here, the products are sodium chloride (NaCl), a neutral salt, and water. The solution resulting from this reaction will have a pH close to 7.
2. Strong Acid-Weak Base Neutralization
When a strong acid reacts with a weak base (e.g., ammonia, NH₃; pyridine, C₅H₅N), the neutralization is not complete. The weak base doesn't fully ionize, leading to the formation of water and a slightly acidic salt. This is because the conjugate acid of the weak base is slightly acidic.
Example:
The reaction between hydrochloric acid (HCl) and ammonia (NH₃) produces ammonium chloride (NH₄Cl), a slightly acidic salt:
HCl(aq) + NH₃(aq) → NH₄Cl(aq)
The resulting solution will have a pH slightly less than 7.
3. Weak Acid-Strong Base Neutralization
Similarly, reacting a weak acid (e.g., acetic acid, CH₃COOH; carbonic acid, H₂CO₃) with a strong base results in incomplete neutralization. This produces water and a slightly basic salt. The conjugate base of the weak acid is slightly basic.
Example:
The reaction between acetic acid (CH₃COOH) and sodium hydroxide (NaOH) yields sodium acetate (CH₃COONa), a slightly basic salt:
CH₃COOH(aq) + NaOH(aq) → CH₃COONa(aq) + H₂O(l)
The resulting solution will have a pH slightly greater than 7.
4. Weak Acid-Weak Base Neutralization
Neutralization reactions between weak acids and weak bases are the most complex. The extent of neutralization depends on the relative strengths of the acid and base. The products are water and a salt, but the pH of the resulting solution is difficult to predict without considering the equilibrium constants of the acid and base. The solution could be acidic, basic, or nearly neutral depending on the specific acid and base involved.
Example:
The reaction between acetic acid (CH₃COOH) and ammonia (NH₃) produces ammonium acetate (CH₃COONH₄), a salt whose solution's pH is closer to neutral.
CH₃COOH(aq) + NH₃(aq) → CH₃COONH₄(aq)
This reaction is less complete than strong acid-strong base reactions and requires more careful analysis to determine the pH of the resulting solution.
The Role of Salts in Neutralization Reactions: A Closer Look
Salts, the ionic compounds formed during neutralization, play a significant role in determining the characteristics of the resulting solution. The pH of the solution depends on the nature of the salt formed:
-
Neutral salts: These are formed from the reaction of a strong acid and a strong base. They do not affect the pH of the solution significantly. Examples include NaCl, KNO₃, and Na₂SO₄.
-
Acidic salts: These are formed from the reaction of a strong acid and a weak base. They have acidic properties due to the presence of the conjugate acid of the weak base. Examples include NH₄Cl and NH₄NO₃.
-
Basic salts: These are formed from the reaction of a weak acid and a strong base. They exhibit basic properties due to the presence of the conjugate base of the weak acid. Examples include CH₃COONa and Na₂CO₃.
-
Amphoteric salts: These salts are capable of acting as both acids and bases. They result from reactions involving weak acids and weak bases or amphiprotic species.
Applications of Neutralization Reactions
Neutralization reactions are ubiquitous in various aspects of our lives and industries. Here are some notable applications:
1. Acid-Base Titrations
Neutralization reactions form the basis of acid-base titrations, a widely used analytical technique to determine the concentration of an unknown acid or base. By carefully measuring the volume of a standard solution (acid or base of known concentration) needed to completely neutralize a given volume of the unknown solution, we can calculate its concentration. This is crucial in various fields, including medicine, environmental monitoring, and industrial chemistry.
2. Industrial Processes
Numerous industrial processes rely on neutralization reactions for controlling pH levels. For example, in wastewater treatment, neutralization is crucial to adjust the pH of wastewater before discharge to prevent environmental damage. In the manufacturing of pharmaceuticals, fine-tuning the pH is vital for stability and efficacy. Neutralization reactions also play a critical role in the production of many salts and other chemicals.
3. Biological Systems
Neutralization reactions are integral to the functioning of biological systems. Our bodies maintain a remarkably stable pH through intricate buffering systems involving neutralization reactions. These systems prevent drastic pH changes that could be detrimental to cellular processes and overall health. For example, the bicarbonate buffer system in our blood helps to regulate blood pH.
4. Everyday Life
Many everyday activities involve neutralization reactions. For example, antacids, which are used to relieve heartburn caused by excess stomach acid, work through neutralization reactions. The active ingredients in antacids are bases that neutralize the stomach acid, thereby alleviating discomfort.
Beyond the Basics: Factors Influencing Neutralization Reactions
Several factors influence the outcome and efficiency of neutralization reactions:
-
Concentration of reactants: Higher concentrations of reactants generally lead to faster and more complete neutralization.
-
Temperature: Increasing the temperature typically increases the rate of reaction.
-
Presence of catalysts: Some catalysts can accelerate neutralization reactions.
-
Equilibrium constants: For weak acids and bases, the equilibrium constants (Ka and Kb) determine the extent of ionization and, consequently, the extent of neutralization.
Conclusion: The Significance of Understanding Neutralization Products
The products of neutralization reactions – water and a salt – hold significant importance in various scientific disciplines and technological applications. Understanding the types of salts formed and their properties, whether neutral, acidic, or basic, is crucial for predicting the characteristics of the resulting solution and controlling the outcome of neutralization processes. From the precise determination of concentrations in analytical chemistry to the maintenance of stable pH levels in biological systems and industrial processes, the principles of neutralization reactions are fundamental to our understanding and manipulation of the chemical world. By mastering these concepts, we can effectively utilize neutralization reactions to address challenges and create innovative solutions across diverse fields.
Latest Posts
Latest Posts
-
Why Is Water Known As Universal Solvent
Mar 21, 2025
-
How Can An Igneous Rock Turn Into A Sedimentary Rock
Mar 21, 2025
-
How To Put Cot In Calculator
Mar 21, 2025
-
What Is The Correct General Equation For Cellular Respiration
Mar 21, 2025
-
What Is The Percentage Of 5 Out Of 25
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Are The Products Of The Neutralization Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
