Why Is Water Known As Universal Solvent
listenit
Mar 21, 2025 · 5 min read
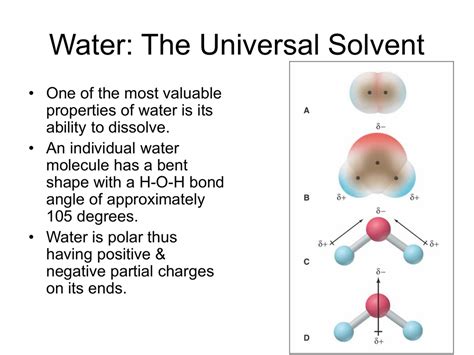
Table of Contents
Why Is Water Known as the Universal Solvent?
Water's reputation as the "universal solvent" is a powerful, albeit slightly misleading, moniker. While it doesn't dissolve everything, its exceptional ability to dissolve a vast array of substances is crucial for life on Earth and numerous industrial processes. This article delves deep into the reasons behind water's remarkable solvent properties, exploring the underlying chemistry and its significant implications.
The Polarity Powerhouse: Understanding Water's Structure
The key to understanding water's solvent prowess lies in its molecular structure. Each water molecule (H₂O) consists of two hydrogen atoms covalently bonded to a single oxygen atom. However, the oxygen atom is significantly more electronegative than the hydrogen atoms, meaning it attracts electrons more strongly. This uneven distribution of charge creates a polar molecule, with a slightly negative charge (δ-) near the oxygen atom and slightly positive charges (δ+) near the hydrogen atoms. This polarity is the cornerstone of water's solvent abilities.
Hydrogen Bonding: The Glue That Holds It Together (and Dissolves Things)
The polarity of water molecules allows them to form hydrogen bonds with each other and with other polar molecules. A hydrogen bond is a relatively weak electrostatic attraction between a slightly positive hydrogen atom and a slightly negative atom (like oxygen, nitrogen, or fluorine) in another molecule. These bonds are individually weak, but collectively they are powerful enough to influence water's physical properties like high boiling point and surface tension. More importantly for our discussion, they are essential for dissolving many substances.
Dissolving the Mystery: How Water Works Its Magic
Water's ability to dissolve substances depends on the interaction between water molecules and the molecules of the solute (the substance being dissolved). Substances that readily dissolve in water are called hydrophilic ("water-loving"), while those that don't are called hydrophobic ("water-fearing").
Dissolving Ionic Compounds: The Dance of Ions
Ionic compounds, like table salt (NaCl), are composed of positively charged ions (cations) and negatively charged ions (anions) held together by strong electrostatic forces. When salt is added to water, the polar water molecules surround the ions. The slightly negative oxygen atoms attract the positive sodium ions (Na+), while the slightly positive hydrogen atoms attract the negative chloride ions (Cl-). This process, called hydration, weakens the ionic bonds holding the salt crystal together, causing the ions to separate and disperse throughout the water, forming a solution.
Dissolving Polar Covalent Compounds: A Similar Attraction
Polar covalent compounds, like sugar (sucrose), have an uneven distribution of charge within their molecules, similar to water. The slightly positive and negative regions of the sugar molecule are attracted to the oppositely charged regions of the water molecules. This attraction allows the sugar molecules to be surrounded by water molecules, dissolving them into the solution. Hydrogen bonding may also play a role in this process, depending on the structure of the polar molecule.
Why "Universal" is a Relative Term: The Limits of Water's Solvency
Despite its impressive solvent power, water's ability to dissolve substances is not unlimited. Many substances, particularly nonpolar molecules like oils and fats, are insoluble in water. This is because nonpolar molecules have no significant charge separation, and thus have no strong attraction to the polar water molecules. Instead of dissolving, these substances tend to clump together, minimizing their contact with water. This phenomenon is known as hydrophobic interaction.
The Importance of Water as a Solvent: A Deeper Dive
Water's solvent properties are fundamental to life and countless applications.
Biological Significance: The Solvent of Life
Water's role as a solvent is paramount in biological systems. It acts as a medium for:
-
Biochemical reactions: Most biochemical reactions occur in aqueous solutions. Water facilitates the interactions between enzymes and substrates, enabling metabolic processes.
-
Nutrient transport: Water transports nutrients and other essential molecules throughout the body of organisms, from the smallest bacteria to the largest whales.
-
Waste removal: Water plays a crucial role in removing metabolic waste products from cells and organisms.
-
Maintaining cellular structure: Water helps maintain the structure and shape of cells, contributing to their overall functionality.
-
Temperature regulation: Water's high heat capacity helps regulate temperature fluctuations in organisms and their environments.
Industrial Applications: A Versatile Solvent
In industry, water's solvent properties are extensively used in:
-
Chemical reactions: Water acts as a solvent and reactant in many chemical processes, from manufacturing pharmaceuticals to producing plastics.
-
Cleaning and purification: Water is used extensively for cleaning and purifying materials, from washing clothes to purifying water supplies.
-
Mining and extraction: Water is used to extract valuable minerals and metals from ores.
-
Food processing: Water is used extensively in food processing, from cleaning and washing to preparing ingredients.
Environmental Impact: Water's Role in Pollution
The ability of water to dissolve so many substances also contributes to pollution. Water readily dissolves pollutants, which then can spread throughout the environment. This makes water pollution a major environmental concern requiring careful management and sustainable practices.
Conclusion: A Remarkable Molecule
Water's exceptional ability to dissolve a wide range of substances, stemming from its polar nature and capacity for hydrogen bonding, is what makes it such a vital component of life and numerous applications. While not truly a "universal" solvent, its remarkable solvency makes it a crucial agent in the world around us, both natural and man-made. Understanding its properties and limitations is paramount to addressing issues related to environmental sustainability, public health, and industrial processes. The seemingly simple water molecule hides a complexity and significance that continues to fascinate and inspire scientific inquiry.
Latest Posts
Latest Posts
-
What Color Is The Coldest Star
Mar 28, 2025
-
Find A Direct Variation Model That Relates Y And X
Mar 28, 2025
-
Least Common Multiple Of 12 And 36
Mar 28, 2025
-
5x 4 5x 4 2 3
Mar 28, 2025
-
How To Find The Charge Of Transition Metals
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Why Is Water Known As Universal Solvent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
